��Ŀ����
����Ŀ����(Ge)�ǰ뵼��Ԫ�أ�Ӧ�ù㷺���ش��������⣺
��1������ΪGe�۵��Ӳ�����Ų�ͼ��ʾ��״̬�У�������ͺ���ߵķֱ�Ϊ____��_____����ѡ���
A.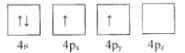 B.
B.
C.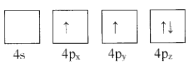 D.
D.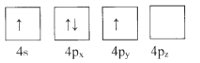
��2��GeH4�Ŀռ乹��Ϊ____���Ƚ���ͬ������⻯��ķе������ʾ��������仯���ɼ�ԭ��____��
CH4 | SiH4 | GeH4 | |
�е�/�� | -161.5 | -119 | -88.1 |
��3���л������A��һ����ҽ�Ʊ������ã���ṹ��ʽΪCF3N=GeH2����Ge���ӻ���ʽΪ____��̼ԭ��������ԭ�ӽ�ϵļ�������Ϊ_____��
��4��Li2GeF6������Ϊ﮵�صĵ���ʣ���Li��Ge��F�縺���ɴ�С��˳��Ϊ_____��
��5��Ge�������£�����ԭ���������AΪ(0��0��0)��BΪ(![]() ��0��
��0��![]() )��DΪ(
)��DΪ(![]() ��
��![]() ��
��![]() )����Cԭ�ӵ��������Ϊ_____��
)����Cԭ�ӵ��������Ϊ_____��
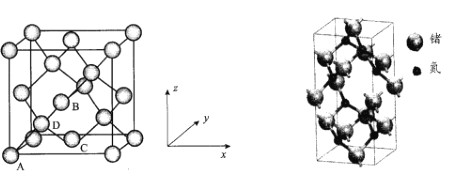
��6�������������ʴ��Ӳ�ȸߵ��ŵ㣬��������ԭ���뵪ԭ��֮��������Ե�s-p�ӻ��������ྦྷ������____���塣һ�ֵ����ྦྷ�������ģ����ͼ��������n(Ge)/n(N)=____�����������������εı߳�Ϊanm�������ӵ�����ֵΪNA��������ܶ�Ϊ��g/cm3������ĸ�Ϊ____nm(�г�����ʽ����
���𰸡�A C �������� ��Ϊ���Ӿ��壬�ҽṹ���ƣ���Է�������Խ��ģ��е�Խ�� sp2 ���������ۼ��� F>Ge>Li (![]() ��
��![]() ��0) ԭ�Ӿ��� 3��4
��0) ԭ�Ӿ��� 3��4 ![]()
��������
(1)����ռ�ݵ��ܼ�Խ�ͣ�����Խ�ͣ�����ռ�ݵ��ܼ�Խ�ߣ�����Խ�ߣ�����ͼʾ��֪��A������ͣ�C������ߣ�
(2)��λ�ڵ������ڵ�IVA�壬��������C���ƣ�GeH4��CH4�ռ�ṹ���ƣ�Ϊ�������壻CH4��SiH4��GeH4���Ƿ��Ӿ��壬�ҽṹ���ƣ���Է�������Խ��ģ��е�Խ�ߣ�
(3)�л������A�Ľṹ��ʽΪCF3N=GeH2��Ge�γ���˫������ƽ��ṹ����Ge���ӻ���ʽΪsp2��̼ԭ��������ԭ�ӽ�ϵļ���Ϊ��ѧ��������������
(4)Li2GeF6������Ϊ﮵�صĵ���ʣ�����Ԫ�������ɣ�Ԫ�����ڱ���F�縺�����ͬ����Խ���ң��縺��Խ��ͬ����Խ���ϣ��縺��Խ����Li��Ge��F�縺���ɴ�С��˳��ΪF>Ge>Li��
(5) D����Χ4��ԭ���γ���������ṹ��D�붥��A�����ߴ��ھ�����Խ����ϣ�������B��C���ϵ�������ԭ�ӵ�ƽ����ƽ�в��潫����2�ȷ֣�ͬ����Dԭ�ӵ���ƽ�в����ƽ�潫���������2�ȷ֣�D���ڵ��������![]() �������Խ��Ge�ľ���ͼ������ԭ���������AΪ(0��0��0)��BΪ(
�������Խ��Ge�ľ���ͼ������ԭ���������AΪ(0��0��0)��BΪ(![]() ��0��
��0��![]() )��DΪ(
)��DΪ(![]() ��
��![]() ��
��![]() )��Cԭ��λ�ڵ�������ģ�Cԭ�ӵ��������Ϊ(
)��Cԭ��λ�ڵ�������ģ�Cԭ�ӵ��������Ϊ(![]() ��
��![]() ��0)��
��0)��
(5)�����������ʴ��Ӳ�ȸߵ��ŵ㣬����ԭ�Ӿ���������������ྦྷ���У�8����ԭ��λ���ڲ���4����ԭ�������ϣ�10����ԭ�������ϣ�����ԭ�ӵĸ����ǣ�![]() ����n(Ge)��n(N)= 3��4���������������εı߳�Ϊanm�������ӵ�����ֵΪNA��������ܶ�Ϊ��g/cm3�����������Ϊ
����n(Ge)��n(N)= 3��4���������������εı߳�Ϊanm�������ӵ�����ֵΪNA��������ܶ�Ϊ��g/cm3�����������Ϊ![]() ������ĸ�Ϊ
������ĸ�Ϊ![]() ��
��

 ȫ�ŵ�����Ԫ�ƻ�ϵ�д�
ȫ�ŵ�����Ԫ�ƻ�ϵ�д�