��Ŀ����
��֪1mol SO2(g)����Ϊ1mol SO3�Ħ�H=-99kJ��mol-1.��ش��������⣺
��1����֪�������ȼ����Ϊ296 KJ��mol-1��������S(s)����3 molSO3(g)�ġ�H =
�����ȼ���(��ͼ)��100 mL 0.50 mol/L��CH3COOH��Һ��100 mL 0.55 mol/L NaOH��Һ��ϣ��¶ȴ�298.0 K���ߵ�300.7 K����֪���ȼƵ����ݳ���(���ȼƸ�����ÿ����1 K����Ҫ������)��150.5 J/K����Һ�ܶȾ�Ϊ1 g/mL��������Һ�ı�����c��4.184 J/(g��K)��
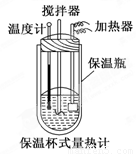
(2) CH3COOH���к��Ȧ�H��_______________________________.
��3��CH3COOH���к��ȵ�����ֵΪ��56.1 kJ/mol������Ϊ(1)�в�õ�ʵ��ֵƫ����ܵ�ԭ���ǣ�����㣩____________________________________________
��1����1185kJ/mol��2�֣� ��2����53.3kJ/mol��2�֣�
��3�������ȼƵı���ƿЧ�����ã��������Һ��ϲ�Ѹ�٣����¶ȼƲ�����ȷ��
��������
�����������1����֪�������ȼ����Ϊ296kJ/mol����ָ1mol����ȫȼ�������ȶ��������������ʱ�ų�������������ȼ�յ��Ȼ�ѧ����ʽΪ����S��s��+O2��g����SO2��g����H����296 kJ/mol���������������Ϊ����������Ȼ�ѧ����ʽΪ����2SO2��g��+O2��g����2SO3��g����H����198KJ/L�����ݸ�˹���ɼ���õ��١�2+�ڣ���2S��s��+3O2��g����2SO3��g�������ԡ�H��[-296��2+��-198��]����790KJ/mol������3mol S��s������3mol SO3��g�����ʱ䣽3��790KJ/mol��2����1185 kJ/mol��
��2��������������Ƶ����ʵ����ֱ���0.05mol��0.055mol������ˮ�����ʵ�����0.05mol����Ӧ�зų��������ǣ�300.7K��298.0K����4.18 J/(g��K)��200g����300.7K��298.0K����150.5 J/K��2663.55J����CH3COOH���к��Ȧ�H����2.66355kJ��0.05mol����53.3kJ/mol
��3��CH3COOH���к��ȵ�����ֵΪ��56.1 kJ/mol�����ⶨ���ƫ��.��˵����Ӧ����������ʧ�����Կ��ܵ�ԭ���Т����ȼƵı���ƿЧ�����ã��������Һ��ϲ�Ѹ�٣����¶ȼƲ�����ȷ�ȡ�
���㣺���鷴Ӧ�ȵļ��㡢�к��Ȳⶨ���й��ж�
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣���������ǿ�����۽̲ģ������ڵ���ѧ����ѧϰ��Ȥ��ѧϰ�����ԣ�Ҳ����������ѧ���淶�Ͻ���ʵ��������������ѧ����ѧ��������

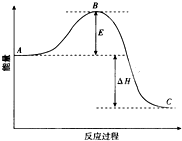 2SO2��g��+O2��g��=2SO3��g����Ӧ���̵������仯��ͼ��ʾ����֪1mol SO2��g������Ϊ1mol SO3�ġ�H=-99kJ?mol-1����ش��������⣺
2SO2��g��+O2��g��=2SO3��g����Ӧ���̵������仯��ͼ��ʾ����֪1mol SO2��g������Ϊ1mol SO3�ġ�H=-99kJ?mol-1����ش��������⣺ 2SO2��g��+O2��g��?2SO3��g����Ӧ���̵������仯��ͼ��ʾ��
2SO2��g��+O2��g��?2SO3��g����Ӧ���̵������仯��ͼ��ʾ�� ��1����4�֣�2SO2��g��+O2��g��?2SO3��g����Ӧ���̵������仯��ͼ��ʾ����֪1mol SO2��g������Ϊ1mol SO3��g���ġ�H=-99kJ/mol��
��1����4�֣�2SO2��g��+O2��g��?2SO3��g����Ӧ���̵������仯��ͼ��ʾ����֪1mol SO2��g������Ϊ1mol SO3��g���ġ�H=-99kJ/mol�� ��1����25�桢101kPa�£�1g������ȫȼ������CO2��Һ̬H2O���ų�55kJ��������д����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ��
��1����25�桢101kPa�£�1g������ȫȼ������CO2��Һ̬H2O���ų�55kJ��������д����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ��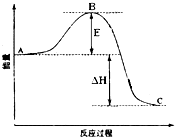 2SO2��g��+O2��g��=2SO3��g����Ӧ���̵������仯��ͼ��ʾ����֪1mol SO2��g������Ϊ1mol SO3�ġ�H=-99kJ?mol-1����ش��������⣺
2SO2��g��+O2��g��=2SO3��g����Ӧ���̵������仯��ͼ��ʾ����֪1mol SO2��g������Ϊ1mol SO3�ġ�H=-99kJ?mol-1����ش��������⣺