��Ŀ����
��13�֣�����ͼ��ʾ���ס������ص缫���϶���������̼������ش��������⣺
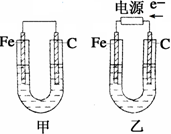
��1���������о�ΪCuSO4��Һ����Ӧһ��ʱ���
���к�ɫ�����������Ǽ׳��е�_____�����ҳ��е�____����
���ҳ��������ĵ缫��Ӧʽ��__ ___��
��2���������о�Ϊ����NaCl��Һ��
��д���ҳ����ܷ�Ӧ�����ӷ���ʽ___ _��
�ڼ׳���̼���ϵ缫��Ӧʽ��__ ____��
�ҳ�̼���ϵ缫��Ӧ����___ ___(�������Ӧ����ԭ��Ӧ��)��
�۽�ʪ���KI������ֽ�����ҳ�̼��������������ֽ������
��Ӧ�Ļ�ѧ����ʽΪ__ ____ __��
�����ҳ�ת��0.02 mol e-��ֹͣʵ�飬�ָ������£�������Һ�����200 mL������Һ��Ͼ��Ⱥ��PH��______��

��1���������о�ΪCuSO4��Һ����Ӧһ��ʱ���
���к�ɫ�����������Ǽ׳��е�_____�����ҳ��е�____����
���ҳ��������ĵ缫��Ӧʽ��__ ___��
��2���������о�Ϊ����NaCl��Һ��
��д���ҳ����ܷ�Ӧ�����ӷ���ʽ___ _��
�ڼ׳���̼���ϵ缫��Ӧʽ��__ ____��
�ҳ�̼���ϵ缫��Ӧ����___ ___(�������Ӧ����ԭ��Ӧ��)��
�۽�ʪ���KI������ֽ�����ҳ�̼��������������ֽ������
��Ӧ�Ļ�ѧ����ʽΪ__ ____ __��
�����ҳ�ת��0.02 mol e-��ֹͣʵ�飬�ָ������£�������Һ�����200 mL������Һ��Ͼ��Ⱥ��PH��______��
��1���� ̼��1�֣���������1�֣� �� 4OH����4e����2H2O��O2����2�֣�
��2���� 2Cl����2H2O Cl2����H2����2OH����2�֣�
Cl2����H2����2OH����2�֣�
�� 2H2O��O2��4e����4OH������2�֣���������Ӧ��1�֣�
�� Cl2��2KI��I2��2KCl����2�֣�
�� 13��2�֣�
��2���� 2Cl����2H2O
 Cl2����H2����2OH����2�֣�
Cl2����H2����2OH����2�֣��� 2H2O��O2��4e����4OH������2�֣���������Ӧ��1�֣�
�� Cl2��2KI��I2��2KCl����2�֣�
�� 13��2�֣�
��

��ϰ��ϵ�д�
�����Ŀ
 +2e
+2e =H
=H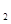 ��
��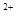 +2e=Fe
+2e=Fe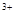 e
e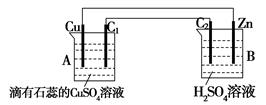

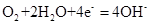
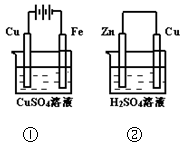
 �õ�صĸ�����ӦʽΪ����������������������������������
�õ�صĸ�����ӦʽΪ����������������������������������
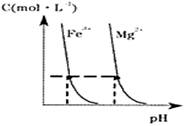
 ����ȷ���� �� ��
����ȷ���� �� ��
 Cu2++2Fe2+
Cu2++2Fe2+