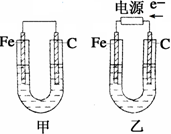
��Ŀ����
����ͼװ�ý���ʵ�飬��֪C1��C2Ϊʯī����
�ش���������:
��1���ж�װ�õ����ƣ�A��Ϊ�ߣߣߣߣߣߣߣ�B��Ϊ�ߣߣߣߣߣߣߣߣ�
��2��п��Ϊ�ߣߣߣߣ����缫��ӦʽΪ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�
ͭ��Ϊ�ߣߣߣߣ����缫��ӦʽΪ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�
ʯī��C1Ϊ�ߣߣ����缫��ӦʽΪ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�
ʯī��C1����������ʵ������Ϊ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��3����C1������224mL���壨��״���£�ʱ��п�������ߣߣߣߣ�����ӡ��������䡱���١����ˣߣߣߣߣ�g��CuSO4��Һ�������ߣߣߣߣ�����ӡ��������䡱���١����ߣߣߣߣ�g��

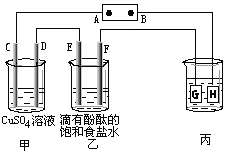
�ش���������:
��1���ж�װ�õ����ƣ�A��Ϊ�ߣߣߣߣߣߣߣ�B��Ϊ�ߣߣߣߣߣߣߣߣ�
��2��п��Ϊ�ߣߣߣߣ����缫��ӦʽΪ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�
ͭ��Ϊ�ߣߣߣߣ����缫��ӦʽΪ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�
ʯī��C1Ϊ�ߣߣ����缫��ӦʽΪ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�
ʯī��C1����������ʵ������Ϊ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��3����C1������224mL���壨��״���£�ʱ��п�������ߣߣߣߣ�����ӡ��������䡱���١����ˣߣߣߣߣ�g��CuSO4��Һ�������ߣߣߣߣ�����ӡ��������䡱���١����ߣߣߣߣ�g��
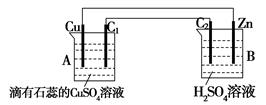
��1������ ԭ���
��2������Zn-2e-=Zn2+������Cu2++2e-= Cu��������4OH--4e-=2H2O+O2�����������ݣ�ͬʱ��Һ��ɫ���
��3�����١�1.3����С��1.6
��2������Zn-2e-=Zn2+������Cu2++2e-= Cu��������4OH--4e-=2H2O+O2�����������ݣ�ͬʱ��Һ��ɫ���
��3�����١�1.3����С��1.6
B��Ϊԭ��أ����Խ�ǿ��п��������ʧ���ӣ�Zn-2e-=Zn2+��̼����������2H����2e��=H2��
A��Ϊ����ʳأ������Դ����������ͭ��Ϊ������Cu2++2e-= Cu
�����Դ����������C1��Ϊ������4OH--4e-=2H2O+O2��
��3���ɹ�ϵʽO2����4e-��2Zn��2CuO��֪����C1������224mL��������״���£�ʱ���μӷ�Ӧ��п��������Ϊ1.3g��CuSO4��Һ���ٵ���������CuO��������1.6g
A��Ϊ����ʳأ������Դ����������ͭ��Ϊ������Cu2++2e-= Cu
�����Դ����������C1��Ϊ������4OH--4e-=2H2O+O2��
��3���ɹ�ϵʽO2����4e-��2Zn��2CuO��֪����C1������224mL��������״���£�ʱ���μӷ�Ӧ��п��������Ϊ1.3g��CuSO4��Һ���ٵ���������CuO��������1.6g

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 NiOOH + MH������������ȷ���ǣ� ��
NiOOH + MH������������ȷ���ǣ� ��