��Ŀ����
6����ҵ�ϳ�ȥCO�Ļ�ѧ��Ӧ����ʽ��HAc��ʾ���ᣩ��Cu��NH3��2Ac+CO+NH3�TCu��NH3��2��CO��Ac����ش��������⣺��1��C��N��O�ĵ�һ�������ɴ�С��˳��ΪN��O��C��
��2��д����̬Cu+�ĺ�������Ų�ʽ1s22s22p63s23p63d10��
��3�������Cu��NH3��3��CO��Ac����ԭ�ӵ���λ��Ϊ4������Cu����Ķѻ���ʽΪ�����������ܶѻ�����λ��Ϊ12��
��4����һ��������NH3��CO2�ܺϳɻ�������[CO��NH2��2]��������Cԭ�Ӻ�Nԭ�ӹ�����ӻ����ͷֱ�Ϊsp2��sp3��1mol���ط����ЦҼ�����ĿΪ7NA��
���� ��1��C��O��NԪ�ض��ǵڶ����ڷǽ���Ԫ�أ�ͬһ����Ԫ��������ҵ�һ�����ܳ��������ƣ���NԪ��ԭ��2p�ܼ��ǰ����ȶ�״̬�������ϵͣ���һ�����ܸ���ͬ��������Ԫ�أ��ʵ�һ������C��O��N���ݴ˴��⣻
��2������CuΪ29��Ԫ�أ����ݵ����Ų�ʽ����д���������
��3�����ݻ�����Ļ�ѧʽ�жϣ�һ��ͭ�������������������һ���ʻ����壬��4�����壻�ڽ�����������ܶѻ��У�����ÿ��ԭ����˵��������Χ��ԭ������֮ͬһ����������ԭ�Ӻ���һ�����������һ�����������ÿ��ԭ����Χ����12��ԭ����֮������
��4�������ӻ�����ʱ���ݵ��Ӷ������жϣ�����ԭ�ӵļ۵�����������������ĺͳ���2�͵õ����Ӷ��������ݵ��Ӷ���������ȷ���ӻ����ͣ�
��� �⣺��1��C��O��NԪ�ض��ǵڶ����ڷǽ���Ԫ�أ�ͬһ����Ԫ��������ҵ�һ�����ܳ��������ƣ���NԪ��ԭ��2p�ܼ��ǰ����ȶ�״̬�������ϵͣ���һ�����ܸ���ͬ��������Ԫ�أ��ʵ�һ������C��O��N���ʴ�Ϊ��N��O��C��
��2��CuΪ29��Ԫ�أ�Ҫע��3d���д��4s�����ǰ�棬ͬʱ���о�������3d�ṹ��Cu+�Ļ�̬�����Ų�ʽΪ1s22s22p63s23p63d10���ʴ�Ϊ��1s22s22p63s23p63d10��
��3��һ��ͭ�������������������һ���ʻ����壬��4�����壬�ڽ�����������ܶѻ��У�����ÿ��ԭ����˵��������Χ��ԭ������֮ͬһ����������ԭ�Ӻ���һ�����������һ�����������ÿ��ԭ����Χ����12��ԭ����֮�������ʴ�Ϊ��4�������������ܶѻ���12��
��4������ԭ��Ϊ̼���۵�����Ϊ4������Ϊ����ԭ�ӣ����ṩ���ӣ�ÿ���ǰ����ṩһ�����ӣ����Ӷ���Ϊ��4+1��2����2=3�����ӻ����Ϊsp2����ԭ���γ���3���Ҽ���ͬʱ����һ�Թµ��ӣ����Ӷ���Ϊ3+1=4�����ӻ����Ϊsp3���Ҽ�����ĿΪ3��ÿ���ǰ����ЦҼ�����Ŀ2��һ���������к��Ҽ�����ĿΪ3+2��2=7����ÿĦ�������к��ЦҼ�����ĿΪ7NA��
�ʴ�Ϊ��sp2��sp3��7NA��
���� ���⿼���˵�һ�����ܵĴ�С�Ƚϣ�һ��ͭ���ӵĺ�������Ų�Ϊ1s22s22p63s23p63d10�����ݻ�����Ļ�ѧʽ�ж�����������ӻ����ͼ��㣬������ԭ�Ӹ������㣮

 ��У����ϵ�д�
��У����ϵ�д�| A�� | ����Fe��Br2��� | B�� | �������NaOH��Һ���� | ||
| C�� | ��ϩ����ˮ��Ӧ | D�� | �������NaOH���Ҵ���Һ���� |
| A�� | S2- | B�� | N3- | C�� | Cl- | D�� | Mg2+ |
| NaCl | MgCl2 | AlCl3 | SiCl4 | |
| �۵�/�� | 801 | 714 | 190 | -70 |
| �е�/�� | 1��413 | 1��412 | 180 | 57.57 |
���Ȼ����ڼ���ʱ��������
�����Ȼ����ھ�̬ʱ���ڷ��Ӿ��壬
���Ȼ��ƾ���������֮���Է��»�����ϣ�
���Ȼ�þ���۷е���Ȼ��Ƶͣ���Ҫ���ܶѻ���ʽ�����ļ��ԡ�Ʒ���ܵ�Ӱ�죮
| A�� | �� | B�� | �ۢ� | C�� | �٢ڢ� | D�� | �٢ڢۢ� |
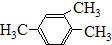
| A�� | 3-��-4-�һ����� | B�� | 2��3-�������� | ||
| C�� | 3��4��4-�������� | D�� | 3��5-�������� |
| A�� | $\stackrel{235}{92}$U��$\stackrel{238}{92}$U����������ͬ��������ͬ��ͬ�ֺ��� | |
| B�� | ͬ��Ԫ�ص�ԭ�Ӿ�����ͬ���������������� | |
| C�� | ������Ϊ53��������Ϊ78�ĵ�ԭ�ӣ�$\stackrel{131}{53}$I | |
| D�� | ͨ����ѧ�仯����ʵ��16O��18O����ת�� |

| A�� | X������һ������M | B�� | ����ӦΪ���ȷ�Ӧ | ||
| C�� | �÷�Ӧһ��Ҫ���Ⱥ���ܷ��� | D�� | ��Ӧ������������������������ |
 D��CH3-CH2-CH2-CH3��
D��CH3-CH2-CH2-CH3��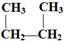 E��Һ�Ⱥ����� F��CO��NH2��2��NH4CNOG��C60�ͽ��ʯ
E��Һ�Ⱥ����� F��CO��NH2��2��NH4CNOG��C60�ͽ��ʯ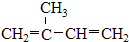 ����ClCH=CHCl��
����ClCH=CHCl�� ����
����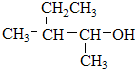 ����
����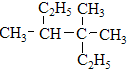 ����
����
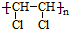 ��
�� ��
��