��Ŀ����
13��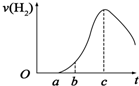 ���ڿ����о��õ���Ƭ5.0gͶ��ʢ��500mL 0.5mol•L-1������Һ���ձ��У�����Ƭ�����ᷴӦ���������������뷴Ӧʱ�䣨��λ���룩�Ĺ�ϵ������ͼ��������ʾ���ش��������⣺
���ڿ����о��õ���Ƭ5.0gͶ��ʢ��500mL 0.5mol•L-1������Һ���ձ��У�����Ƭ�����ᷴӦ���������������뷴Ӧʱ�䣨��λ���룩�Ĺ�ϵ������ͼ��������ʾ���ش��������⣺��1��������O��a�β�����������ԭ�� �����йط�Ӧ�Ļ�ѧ����ʽ��ʾ����Al2O3+6H+=2Al3++3H2O��
��2������a��c�Σ��������������ʼӿ��ԭ���Ƿ�Ӧ���ȣ���Һ�¶����ߣ���Ӧ���ʼӿ�
��3����b��c���ʱ��Ϊ6min�����������Һ��Ũ����0.45mol•L-1����0.15mol•L-1�������ʱ�����������ʾ��ƽ������Ϊ0.05mol•L-1min-1�����跴Ӧǰ����Һ������䣩��
��4��Ϊ�˼���������Ӧ�����ʣ��������Һ�м����������ʣ�����Ϊ���е���ACE������ĸ����
A������ˮ B������ع��� C����������ҺD����������Ũ���� E�������¶� F������Ƭ��Ϊ���ۣ�
���� ��1���������ı�����һ�����ܵ�����Ĥ��
��2�����ݷ�ӳ������Ҫ���ȣ�
��3����v=$\frac{��c}{��t}$���㣻
��4������Ӱ�컯ѧ��Ӧ���ʵ����أ�
��� �⣺��1�������ı�����һ�����ܵ�Al2O3����HCl��Ӧ�õ��κ�ˮ���������ų����ʴ�Ϊ��Al2O3+6H+=2Al3++3H2O��
��2���ڷ�Ӧ�����У�Ũ�ȼ�С����Ӧ���ʼ�С������Ӧ���ȣ���Һ�¶����ߣ���Ӧ���ʼӿ죬�Һ���Ϊ��Ҫ���أ�
�ʴ�Ϊ����Ӧ���ȣ���Һ�¶����ߣ���Ӧ���ʼӿ죻
��3��v=$\frac{��c}{��t}$=$\frac{0.45mol/L-0.15mol/L}{6min}$=0.05 mol•L-1 min-1���ʴ�Ϊ��0.05 mol•L-1 min-1��
��4��A����������ˮ�����Ũ�ȼ��٣���Ӧ���ʼ�������A��ȷ��
B����������ع��壬��Ӱ��������Ũ�ȣ��Է�Ӧ������Ӱ�죬��B����
C��������������Һ�����Ũ�ȼ��٣���Ӧ���ʼ�������C��ȷ��
D����������Ũ���ᣬ�����ӵ�Ũ������Ӧ���ʼӿ죬��D����
E�������¶ȣ���Ӧ���ʼ�������E��ȷ��
F���������ۣ�����ı�������Ӧ���ʼӿ죬��F����
�ʴ�Ϊ��ACE��
���� ������Ҫ������Ӱ�컯ѧ��Ӧ���ʵ������Լ����㣬�����ں��˻�ѧʵ�飬��ʽ��ӱ��������״����ǵڣ�4��С��ѡ��D��

 ��У����ϵ�д�
��У����ϵ�д�| A�� | ��ϩʹ����KMnO4��Һ��ɫ | |
| B�� | ����������ˮ�У���ˮ��ӽ���ɫ | |
| C�� | ��ϩʹ��ˮ��ɫ | |
| D�� | ������������ϣ�����һ��ʱ������ɫ��dz |
| A�� | 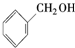 ����-OH ����-OH | B�� | 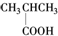 ����-COOH ����-COOH | ||
| C�� | 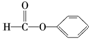 ȩ��-CHO ȩ��-CHO | D�� | CH3-O-CH3������-CHO |
| A�� | 1.4 g | B�� | 15.4 g | C�� | 4.4 g | D�� | 12.4 g |
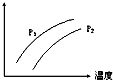 ��ͼ��ʾ�¶ȡ�ѹǿ�Դ�ƽ��Ŀ��淴Ӧ��2L��g��?2M��g��+N��g����H��0��Ӱ�죨P1��P2��ͼ��y���ʾ�������ǣ�������
��ͼ��ʾ�¶ȡ�ѹǿ�Դ�ƽ��Ŀ��淴Ӧ��2L��g��?2M��g��+N��g����H��0��Ӱ�죨P1��P2��ͼ��y���ʾ�������ǣ�������| A�� | �������L�İٷֺ��� | B�� | ���������ܶ� | ||
| C�� | L��ת���� | D�� | ��������ƽ�������� |
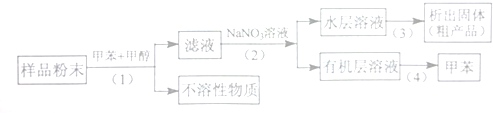
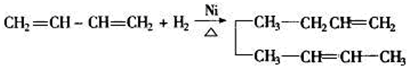
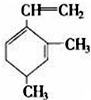 ������������ʵ�����Br2��ˮ��Һ�����ӳɷ�Ӧʱ�ж��ֲ���벹д�����������ֽṹ����������ֲ���Ľṹ��ʽ��
������������ʵ�����Br2��ˮ��Һ�����ӳɷ�Ӧʱ�ж��ֲ���벹д�����������ֽṹ����������ֲ���Ľṹ��ʽ��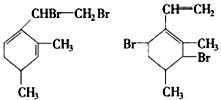
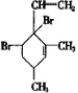
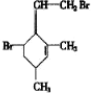
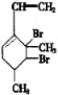 ��
��