题目内容
【题目】化工生产是指对原料进行化学加工,最终获得有价值的产品的生产过程。某研究小组利用含硫酸亚铁和硫酸铜的工业废料制备铁红(氧化铁)和硫酸铵晶体。流程如图:
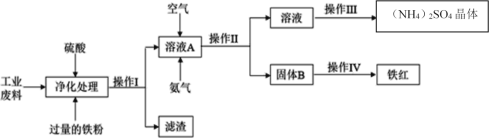
请回答下列问题:
(1)操作I的名称_____,操作Ⅲ的名称____。
(2)滤渣的成分为_____。
(3)请写出溶液A中反应的离子方程式___。在标准状况下,转移0.4NA个电子时,通入的空气体积为_____。
(4)测定废料中硫酸铜的质量分数:称取ag废料样品,将操作I得到的滤渣用足量的稀硫酸溶解、过滤、洗涤、干燥,称得固体的质量为b g,则废料中硫酸铜的质量分数____(写出表达式)。
(5)向工业废料溶液中加入足量的酸性高锰酸钾,写出有关的离子方程式___。
【答案】过滤 蒸发浓缩、冷却结晶 Fe、Cu 10H2O+8NH3+O2+4Fe2+=4Fe(OH)3↓+8NH4+ 10.7L (5b/2a)×100% 5Fe2++MnO4-+8H+=5Fe3++Mn2++4H2O
【解析】
向含硫酸亚铁和硫酸铜的工业废料中加入硫酸溶解,加入过量的铁粉与硫酸铜发生置换反应得到不溶的铜单质,过滤,滤渣为铜和未反应完的铁,溶液A为硫酸亚铁的溶液,向其中通入空气氧化亚铁离子为铁离子,加入氨气沉淀铁离子,过滤,固体B为氢氧化铁,溶液为硫酸铵溶液,将硫酸铵溶液蒸发浓缩、冷却结晶得到硫酸铵晶体,将固体B(氢氧化铁)灼烧得到氧化铁。据此解答。
(1)经过操作I得到固体和溶液,故操作I为过滤;操作Ⅲ为将硫酸铵溶液蒸发浓缩冷却结晶为硫酸铵晶体。故答案为:过滤;蒸发浓缩、冷却结晶;
(2)通过分析,铁粉将硫酸铜还原为铜单质,故滤渣为铜和未反应完的铁;故答案为:Fe、Cu;
(3)溶液A为硫酸亚铁的溶液,向其中通入空气氧化亚铁离子为铁离子,加入氨气沉淀铁离子,过滤,固体B为氢氧化铁,溶液为硫酸铵溶液,溶液A中反应的离子方程式为10H2O+8NH3+O2+4Fe2+=4Fe(OH)3↓+8NH4+。根据反应方程式可得,消耗1mol氧气,转移4mol电子,在标准状况下,转移0.4NA个电子时,需要消耗0.1mol的氧气,体积为2.24L,氧气在空气中的体积分数为21%,因此通入的空气体积为![]() ≈10.7L;答案为:10H2O+8NH3+O2+4Fe2+=4Fe(OH)3↓+8NH4+;10.7L;
≈10.7L;答案为:10H2O+8NH3+O2+4Fe2+=4Fe(OH)3↓+8NH4+;10.7L;
(4)称取ag废料样品,操作I得到的滤渣为铜和铁,用足量的稀硫酸溶解、过滤、洗涤、干燥,称得固体的质量为bg,铜不溶解,故m(Cu)=bg,则n(Cu)=![]() mol,根据铜元素质量守恒,则n(CuSO4)=n(Cu)=
mol,根据铜元素质量守恒,则n(CuSO4)=n(Cu)=![]() mol,则废料中硫酸铜的质量分数为
mol,则废料中硫酸铜的质量分数为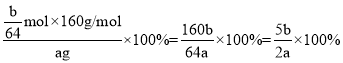 ;故答案为:
;故答案为:![]() ;
;
(5)向工业废料(含硫酸亚铁和硫酸铜)溶液中加入足量的酸性高锰酸钾,硫酸亚铁与酸性高锰酸钾发生氧化还原反应,离子方程式为5Fe2++MnO4-+8H+=5Fe3++Mn2++4H2O;故答案为:5Fe2++MnO4-+8H+=5Fe3++Mn2++4H2O。

 智慧小复习系列答案
智慧小复习系列答案