��Ŀ����
����Ŀ���Ȼ���ͭ��CuCl����ɫ���ױ�������Ksp=1.2��10-6���㷺�����������ѳ�������ɫ���ȡ���ҵ���ó���ͭ��ۣ���Ҫ��Cu2S��CuS��Fe2O3��FeO�ȣ��Ʊ�����CuCl���������£�

��1����������Fe(OH)3�͵�����Ļ�����Ӧ����Cu2S���뷴Ӧ�Ļ�ѧ����ʽΪ��Cu2S+MnO2 + H2SO4��CuSO4 +S+MnSO4 + H2O��δ��ƽ������������Ϊ��__________��
��2����Mn2+ʱ��MnCO3�������÷�Ӧ�����ӷ���ʽΪ_________________________��
��3����֪��Cu(OH)2�����ڰ�ˮ�γ�����ɫ��Һ��Cu(OH)2+4NH3![]() [Cu(NH3)4]2++2OH������������������Ч�����±���
[Cu(NH3)4]2++2OH������������������Ч�����±���
��� | �¶�/�� | ʱ��/min | ѹǿ/KPa | ��Һ��ɫ |
a | 110 | 60 | 101.3 | dz��ɫ |
b | 100 | 40 | 74.6 | ������dz |
c | 90 | 30 | 60.0 | ��ɫ�� |
�ɱ�����Ϣ��֪����������Ӧѡ_______������ţ�������ƽ���ƶ�ԭ������ѡ���������ԭ����__________________________________________________��
��4����Ӧ�������ӷ���ʽ___________________________��
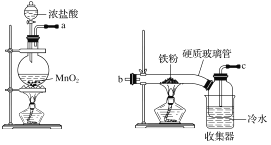
��5��ʵ��֤��ͨ����ͼװ��Ҳ�ɻ��CuCl������Ϊ������������ɫ���壻�������а�ɫ��״���������������ࣻU�ιܵײ���������������ɫ��״������ת��Ϊ����ɫ������
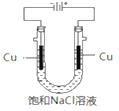
������CuCl�ĵ缫��ӦʽΪ________________________________��
����ͬѧ���������ɫ����������CuOH�������Ǵ������в��ĵ�CuOH���й���Ϣ����Щ����֧�ָ�ͬѧ��˵��________��������ţ�
a��������ˮ��ɺ�ɫ��Cu2O
b��CuOH�ǻ�ɫ��ɫ���壬������ˮ
c��CuOH��Ksp=2��10-15
d
���𰸡�CuSO4��S Mn2+ +HCO+NH3��H2O===MnCO3��+NH+H2O c ��Сѹǿ��ƽ�������ƶ����������� SO2 +2Cu2++2Cl+2H2O===2CuCl��+SO![]() +4H+ Cu-e+Cl===CuCl�� b c
+4H+ Cu-e+Cl===CuCl�� b c
��������
�Գ���ͭ��ۣ���Ҫ��Cu2S��CuS��Fe2O3��FeO�ȣ��Ʊ��Ȼ���ͭ��·��Ϊ���ڷ�ӦI����ͭ����м���������Һ���������̣�����������ԭ��Ӧ�������ʣ��������̱���ԭΪ����������������Һ�У���Һ�к���ͭ���ӡ������ӣ�����Һ�м��백ˮ������������Ϊ���������������ˣ���������Fe��OH��3�͵�����Ļ���Ȼ����Һ�м���̼����麟������ӣ�����������ת��Ϊ̼���̳�����Ȼ�������õ�������ͭ�����������ֽⷴӦ�õ�CuO���塢�����������̼�����������ܽ�CuO�õ�CuCl2��ͨ�������������õ��Ȼ���ͭ�������Դ˽�������
��1��Cu2S���뷴Ӧ�Ļ�ѧ����ʽΪ��Cu2S+MnO2+H2SO4��CuSO4+S+MnSO4+H2O���ڸ÷�Ӧ�У�MnԪ�صĻ��ϼ���+4�۽���Ϊ+2����MnSO4Ϊ��ԭ���ͭԪ�ػ��ϼ���Cu2S�е�+1�����ߵ�CuSO4�е�+2����SԪ�صĻ��ϼ���Cu2S�е�-2������������S�е�0������CuSO4��SΪ��������ʴ�Ϊ��CuSO4��S��
��2����Mn2+ʱ���백ˮ��NH4HCO3��MnCO3��������Ӧ�����ӷ���ʽΪ��Mn2++HCO3-+NH3H2O=MnCO3��+NH4++H2O���ʴ�Ϊ��Mn2++HCO3-+NH3H2O=MnCO3��+NH4++H2O��
��3���������̣�����ʱ[Cu(NH3)4]2+�ȱ��Cu(OH)2�����ݱ�����Ϣ��c�õ��IJ�Һ��ɫΪ��ɫ����˵��[Cu(NH3)4]2+��ת���ʺܴ�����Ч���Ϻ�������������Ӧѡc��ѡ���������ԭ���ǣ���Сѹǿ��ƽ��Cu(OH)2+4NH3![]() [Cu(NH3)4]2++2OH�������ƶ��������������ʴ�Ϊ��c����Сѹǿ��ƽ�������ƶ�������������
[Cu(NH3)4]2++2OH�������ƶ��������������ʴ�Ϊ��c����Сѹǿ��ƽ�������ƶ�������������
��4����Ӧ��Ϊ��CuCl2��Һ��ͨ����������Ƶ�CuCl��CuCl2����ԭ��CuCl��SO2��������SO42-�����ӷ���ʽΪ��SO2+2Cu2++2Cl-+2H2O=2CuCl��+SO42-+4H+���ʴ�Ϊ��SO2+2Cu2++2Cl-+2H2O=2CuCl��+SO42-+4H+��
��5���ٸõ���������ˮ�е������ӵõ��ӣ�������ͭ�缫ʧȥ���ӽ������������CuCl����������CuCl�ĵ缫��ӦʽΪ��Cu-e-+Cl-=CuCl����
�ʴ�Ϊ��Cu-e-+Cl-=CuCl����
�ڿ�����������ɫ��״������ת��Ϊ����ɫ����������ɫ����������CuOH��˵��CuOH�ǻ�ɫ��ɫ���壬������ˮ��Ksp��CuCl��=1.2��10-6��Ksp��CuOH��=2��10-15��˵��CuOH���ܽ�Ƚ�֮CuCl���ܽ�ȸ�С������ΪCuCl����ת��ΪCuOH������
�ʴ�Ϊ��bc��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ�������ˮ��Һ�д��ڵ���ƽ�⡢ˮ��ƽ�⡢�ܽ�ƽ�⣬��ش��������⡣
��1����֪��������ĵ��볣�����±���
���� | HCOOH | HCN | H2CO3 |
���볣��(25��) | Ka = 1��77��10 -4 | Ka=4.3��l0-10 | Ka1=5.0��l0-7 Ka2=5.6��l0-11 |
��0.1 moI/L NaCN��Һ��0.1mol/L NaHCO3��Һ�У�c��CN-��______c��HCO3 -��������>������<������=������
�ڳ����£�pH��ͬ��������Һ
A��HCOONa B��NaCN C��Na2CO3��
�����ʵ���Ũ���ɴ�С��˳����________�����ţ���
����֪25��ʱ��HCOOH( aq) +OH -( aq)=HCOO-(aq) +H2O��1�� ��H=-a kJ/mol
H+(aq) +OH-(aq) =H2O��1�� ��H=-b kJ/mol
���������Ȼ�ѧ����ʽΪ__________________________________��
�ܽ�����CO2ͨ��NaCN��Һ����Ӧ�����ӷ���ʽ��______________________��
�������£�����Ũ�ȵ�HCOONa��ҺpH =9�������ӷ���ʽ��ʾ��Һ�ʼ��Ե�ԭ����:
______________________________����Һ��![]() =___________��
=___________��
��2�������£���0.100 mol/L������Һ�ζ�20.00mL0.l00mol/L ��ij��ˮ��Һ���ζ�������ͼ��ʾ��

��d����ʾ����Һ������Ũ���ɴ�С��˳������Ϊ_______________��
��b����ʾ����Һ��c(NH3��H2O)-c(NH4+)=_____������Һ�е���������Ũ�ȱ�ʾ����
��pH =10�İ�ˮ��pH =4��NH4C1��Һ�У���ˮ�������c(H+)֮��Ϊ____��
��3����֪Ksp(BaCO3) =2.6��l0-9��Ksp( BaSO4)=1.1��10-10.
���ֽ�Ũ��Ϊ2��10-4mol/LNa2CO3��Һ��BaCl2��Һ�������ϣ�������BaCO3��������BaCl2��Һ����СŨ��Ϊ____mol/L��
������BaSO4�������Һ�еμ�Na2CO3��Һ������BaCO3��������ʱ����Һ��![]() =___________��������λ��Ч���֣���
=___________��������λ��Ч���֣���
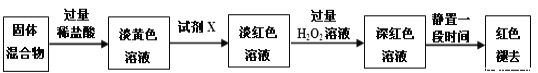
����Ŀ����������ƾ���(Na2S2O3��5H2O)�ֳƺ���������ɫ��״��������ˮ���������Ҵ�����������֯��Ư������ȼ������������еĻ�ԭ����
��. Na2S2O3��5H2O���Ʊ�
Na2S2O3��5H2O���Ʊ������ж��֣������������Ʒ��ǹ�ҵ��ʵ�����е���Ҫ������
Na2SO3+S+5H2O![]() Na2S2O3��5H2O
Na2S2O3��5H2O
�Ʊ��������£�
�ٳ�ȡ12.6gNa2SO3��100mL�ձ��У���50mLȥ����ˮ�����ܽ⡣
����ȡ4.0g�����200mL�ձ��У���6mL�Ҵ���ֽ�����Ƚ�����ʪ���ټ���Na2SO3��Һ����ʯ����С�������У����Ͻ�������ۼ���ȫ����Ӧ��
��ֹͣ���ȣ�����Һ����ȴ���2g����̿���������2����(��ɫ)��
�ܳ��ȹ��ˣ�����Һ���������У�_________________��____________________��
�ݹ��ˡ�ϴ�ӣ�����ֽ���ɺ��أ�������ʡ�
��1�������������Ҵ���ʪ��Ŀ����____________________________��
��2������ܳ��ȹ��˵�ԭ��_____________________���ո�Ӧ��ȡ�IJ�����_________________��____________________��
��3�������ϴ�ӹ����У�Ϊ��ֹ�в��ֲ�Ʒ��ʧ��Ӧѡ�õ��Լ�Ϊ__________________________��
��4����Һ�г�Na2S2O3 ��δ��Ӧ��ȫ��Na2SO3�⣬����ܴ��ڵ���������________________�����ɸ����ʵ�ԭ�������____________________________��
��.��Ʒ���ȵIJⶨ
ȷ��ȡ1.00g��Ʒ(��������ƾ����Ħ������Ϊ248g/mol)������������ˮ�ܽ⣬�Ե�����ָʾ������0.1000mol/LI2�ı���Һ�ζ�����Ӧ�����ӷ���ʽΪ��2S2O32-+I2=S4O62-+2I-����¼�������£�
�ζ����� | �ζ�ǰ����(mL) | �ζ������(mL) |
1 | 0.30 | 21.32 |
2 | 0.36 | 22.56 |
3 | 0.10 | 21.08 |
��5���������ò�Ʒ�Ĵ���Ϊ___________(������λ��Ч����)�������ݵĺ������Ϳ�����__________(������ʵ�������������)��
��.��Ʒ��Ӧ��
��6��Na2S2O3 ���������ȼ�������Һ���ױ�Cl2 ����ΪSO42-���÷�Ӧ�����ӷ���ʽΪ _____________________________��
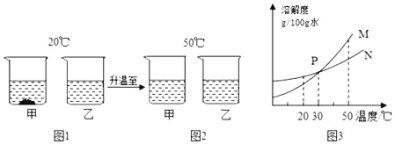
����Ŀ��I������[KAl(SO4)2��12H2O] ���������������й㷺��;������ˮ�ľ�������ֽ��ҵ����ʩ������ʳƷ��ҵ�ķ��ͼ��ȡ������������ķ�����������(��Al�� Al2O3������SiO2��FeO ��xFe2O3)���Ʊ������������������£�

��֪�����������������pH���±���ʾ��
Al��OH��3 | Fe��OH��2 | Fe��OH��3 | |
��ʼ���� | 3.4 | 6.3 | 1.5 |
��ȫ���� | 4.7 | 8.3 | 2.8 |
�ش��������⣺
��1��������ˮ��ԭ����______________________________(�����ӷ���ʽ��ʾ)��
��2����������_______________��_________________�����ˡ�ϴ�ӡ����
��3��������ҺA���Ƿ����Fe2���ķ���______________________________ (ֻ��һ���Լ�)��
��4������ҺA�м��������ط�����Ӧ�����ӷ���ʽΪ��__________��
��5������pH��3��Ŀ����_______________________ ������2���е�������______��
II����6��ȡһ��������Pb2+��Cu2+�Ĺ�ҵ��ˮ�������еμ�Na2S��Һ����PbS��ʼ����ʱ����Һ��c(Pb2+)/c(Cu2+) =_________ (������λ��Ч����)��[��֪Ksp(PbS)=3.4��10-28��
Ksp(CuS)=1.3��10-36]