��Ŀ����
3��ij�¶��£�H2��g��+CO2��g��?H2O��g��+CO��g����ƽ�ⳣ��K=2.25�����¶����ڼס��ҡ������������ܱ������У�Ͷ��H2��g����CO2��g��������ʼŨ�������ʾ��| ��ʼŨ�� | �� | �� | �� |
| c��H2��/mol/L | 0.010 | 0.020 | 0.020 |
| c��CO2��/mol/L | 0.010 | 0.010 | 0.020 |
| A�� | ��Ӧ��ʼʱ����Ӧ���ʣ��ף��ң��� | |
| B�� | ƽ��ʱ�����кͱ���H2��ת���ʾ���60% | |
| C�� | ƽ��ʱ��c��CO2����=2c��CO2���� | |
| D�� | ƽ��ʱ������CO2�������������60% |
���� A������Ũ��Խ��Ӧ����Խ�������ѧ��Ӧ���ʴ�С��
B���÷�Ӧ���������ǰ��ķ�Ӧ�����Լͱ�Ϊ��Чƽ�⣻
C���÷�Ӧ���������ǰ��ķ�Ӧ�����Լͱ�Ϊ��Чƽ�⣬��������ʼŨ��Ϊ������������ƽ��ʱ����Ũ��Ϊ��������
D������ƽ�ⳣ������ƽ��ʱ������̼����������������������൱���Ǽ�������������H2��CO2�����������С���ݴ��жϣ�
��� �⣺A������Ũ��Խ��Ӧ����Խ���֪����Ӧ��ʼʱ�����еķ�Ӧ��Ũ��������Է�Ӧ������죬���еķ�Ӧ��Ũ����С�����Է�Ӧ�������������ԣ���Ӧ���ʣ��ף��ң�������A��ȷ��
B�����������ƽ��ʱ��Ӧ����������Ũ��Ϊxmol/L������K=$\frac{x{\;}^{2}}{��0.01-x��{\;}^{2}}$=2.25����x=0.006������H2��ת������60%���ּͱ�Ϊ��Чƽ�⣬���Լ��кͱ���H2��ת���ʾ���60%����B��ȷ��
C���ͱ�Ϊ��Чƽ�⣬��������ʼŨ��Ϊ������������ƽ��ʱ����Ũ��Ϊ����������C��ȷ��
D�����������ƽ��ʱ��Ӧ���Ķ�����̼��Ũ��Ϊxmol/L������K=$\frac{x{\;}^{2}}{��0.01-x��{\;}^{2}}$=2.25����x=0.006������CO2���������Ϊ$\frac{0.006}{0.01+0.01}$=30%�������������൱���Ǽ�������������H2��CO2�����������С������С��30%����D����
��ѡD��
���� ������Ҫ�����˻�ѧƽ�����ؼ��㡢Ӱ�컯ѧ��Ӧ���ʵ����ء���Чƽ���˼���֪ʶ�㣬�е��Ѷȣ�����ʱҪע��ȽϷ�Ӧǰ����������ı仯������

 �ƸԺ���ȫ�����Ų��Ծ�ϵ�д�
�ƸԺ���ȫ�����Ų��Ծ�ϵ�д�| A�� | ��ҩ��ȡ�÷�ĩ״��С����״���� | |
| B�� | �ý�ͷ�ιܵμ�����Һ�� | |
| C�� | ��ʢ��2/3���Һ����Թܼ��� | |
| D�� | ����ʱ©�����¶˹ܿ�Ҫ�����ձ��ڱ� |
| ������ | ���� | ���� | ������ | |
| ������ | 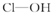 | 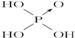 |  |  |
| ���ǻ���ԭ���� | 0 | 1 | 2 | 3 |
| ���� | ���� | ��ǿ�� | ǿ�� | ��ǿ�� |
 ��������
�������� ��
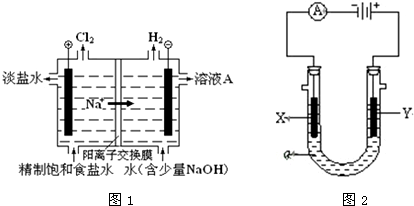
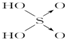
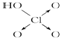
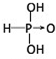
����2���ֱ�д����������������������NaOH��Һ��Ӧ�Ļ�ѧ����ʽ�������H3PO3+2NaOH=Na2HPO3+2H2O�������H3AsO3+3NaOH=Na3AsO3+3H2O��
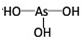
��3���Ƚ�H3AsO4��H2CrO4��HMnO4������ǿ��������������ǿ��˳��H3AsO4��H2CrO4��HMnO4��
��1�����д�ʩ�У������ڽ��ʹ�����CO2Ũ�ȵ���abc��������ĸ��ţ�
a�����ý��ܼ��������ٻ�ʯȼ�ϵ�����
b�������������������У�������̼����
c������̫���ܡ����ܵ�������Դ�����ʯȼ��
��2����һ��;���ǽ�CO2ת�����л���ʵ��̼ѭ�����磺
2CO2��g��+2H2O��l���TC2H4��g��+3O2��g����H=+1411.0kJ/mol
2CO2��g��+3H2O��l���TC2H5OH��1��+3O2��g����H=+1366.8kJ/mol
������ϩˮ�����Ҵ����Ȼ�ѧ����ʽ�ǣ�C2H4��g��+H2O��l��=C2H5OH��l����H=-44.2kJ/mol��
��3����һ�������£�6H2��g��+2CO2��g��?CH3CH2OH��g��+3H2O��g����
| �¶ȣ�k�� CO2ת���ʣ�%�� n��H2��/n��CO2�� | 500 | 600 | 700 | 800 |
| 1.5 | 45 | 33 | 20 | 12 |
| 2 | 60 | 43 | 28 | 15 |
| 3 | 83 | 62 | 37 | 22 |
���¶�һ��ʱ�������̼�ȣ�CO2��ת���������������С�����䡱����
�ڸ÷�Ӧ������ӦΪ�ţ�������š����ȷ�Ӧ��
����ͼһ������ϵ����ͼ��˵��ѹǿ��p1����p2ʱ������ƽ���ƶ�����H2ת���ʺ��Ҵ��ٷֺ����ı仯��

| A�� | 1mol Na2O2��ˮ��Ӧ��ת�Ƶ���1mol | |
| B�� | Na2O��Na2O2���Ԫ����ͬ����CO2��Ӧ�IJ���Ҳ��ͬ | |
| C�� | ������������ˮ��Ӧ���Ʊ�¶�ڿ��������յIJ�����NaOH | |
| D�� | ��0.01mol��Na2O��Na2O2�ֱ�Ͷ��ͬ������ˮ�У����õ�����������������ͬ |
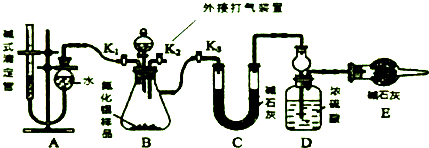
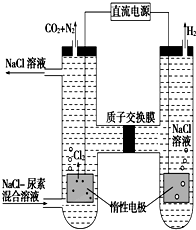 �˹�������ü�ӵ绯ѧ������ȥ��л�����е����أ�CO��NH2��2����ԭ����ͼ��
�˹�������ü�ӵ绯ѧ������ȥ��л�����е����أ�CO��NH2��2����ԭ����ͼ�� Na2O2��ˮ�ķ�Ӧʵ����Na2O2+2H2O�T2NaOH+H2O2����Ӧ�ų�������ʹ����H2O2���ȷֽ⣺2H2O2�T2H2O+O2����Ϊ�˲ⶨij�������ƹ���Ĵ��ȣ���������ʵ�飺
Na2O2��ˮ�ķ�Ӧʵ����Na2O2+2H2O�T2NaOH+H2O2����Ӧ�ų�������ʹ����H2O2���ȷֽ⣺2H2O2�T2H2O+O2����Ϊ�˲ⶨij�������ƹ���Ĵ��ȣ���������ʵ�飺