��Ŀ����
14��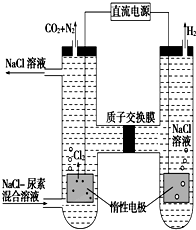 �˹�������ü�ӵ绯ѧ������ȥ��л�����е����أ�CO��NH2��2����ԭ����ͼ��
�˹�������ü�ӵ绯ѧ������ȥ��л�����е����أ�CO��NH2��2����ԭ����ͼ����1���������з����ķ�Ӧ�����У�6Cl--6e-�T3Cl2����CO��NH2��2+3Cl2+H2O�TN2+CO2+6HCl��
��2������������������Һ��pH����ǰ��Ƚ����䣻���������ռ�������13.44L����״���������ȥ������Ϊ7.2g������������ܽ⣬��ͬ����
��3�����ر����²������ϲ���Һ�ĵ��뵼��������������ת����2mol���ӣ������ӽ���Ĥ������Һ�������仯���m��-��m����Ϊ26g��
���� ��1��������ӦΪ��6H2O+6e-�T6OH-+3H2������6H++6e-�T3H2������������ӦΪ��6Cl--6e-�T3Cl2����CO��NH2��2+3Cl2+H2O�TN2+CO2+6HCl��
��2��������ӦΪ��6H2O+6e-�T6OH-+3H2������6H++6e-�T3H2����������ӦΪ��6Cl--6e-�T3Cl2����CO��NH2��2+3Cl2+H2O�TN2+CO2+6HCl������������Ӧʽ���Կ��������������ϲ�����OH-��H+����Ŀ��ȣ��������з�Ӧ������H+ͨ�����ӽ���Ĥ������������OH-ǡ�÷�Ӧ����ˮ�������������е��ǰ����Һ��pH���䣻���������ռ�������13.44L����״����������������ʵ���=$\frac{13.44L}{22.4L/mol}$=0.6mol������CO��NH2��2+3Cl2+H2O�TN2+CO2+6HCl��֪n��N2��=n��CO2��=0.6mol��$\frac{1}{5}$=0.12 mol������Nԭ���غ�������ص����ʵ������ٸ���m=nM��������������
��3��ת��2mol����ʱ������6Cl--6e-�T3Cl2����CO��NH2��2+3Cl2+H2O�TN2+CO2+6HCl��������ӦΪ��6H++6e-�T3H2�����������ӽ���Ĥ��ߵ�2mol�����ӽ�ȥ�ұߣ��Ҳ������ӷų��������������䣻���������������1molCl2�����շų�$\frac{1}{3}$molN2��$\frac{1}{3}$molCO2���ݴ˷�����
��� �⣺��1������ͼ֪��������ӦΪ��6H2O+6e-�T6OH-+3H2������6H++6e-�T3H2������������ӦΪ��6Cl--6e-�T3Cl2����CO��NH2��2+3Cl2+H2O�TN2+CO2+6HCl��
�ʴ�Ϊ��6Cl--6e-�T3Cl2����CO��NH2��2+3Cl2+H2O�TN2+CO2+6HCl��
��������ӦΪ6H2O+6e-�T6OH-+3H2������6H++6e-�T3H2������������ӦΪ6Cl--6e-�T3Cl2����CO��NH2��2+3Cl2+H2O�TN2+CO2+6HCl������������Ӧʽ���Կ��������������ϲ�����OH-��H+����Ŀ��ȣ��������з�Ӧ������H+ͨ�����ӽ���Ĥ������������OH-ǡ�÷�Ӧ����ˮ�������������е��ǰ����Һ��pH���䣻���������ռ�������13.44L����״����������������ʵ���=$\frac{13.44L}{22.4L/mol}$=0.6mol������CO��NH2��2+3Cl2+H2O�TN2+CO2+6HCl��֪n��N2��=n��CO2��=0.6mol��$\frac{1}{5}$=0.12 mol������Nԭ���غ��n[CO��NH2��2]=n��N2��=0.12mol����������m=nM=0.12mol��60g/mol=7.2g��
�ʴ�Ϊ�����䣻7.2��
��3��ת��2mol����ʱ������6Cl--6e-�T3Cl2����CO��NH2��2+3Cl2+H2O�TN2+CO2+6HCl��������ӦΪ��6H++6e-�T3H2�����������ӽ���Ĥ��ߵ�2mol�����ӽ�ȥ�ұߣ��Ҳ������ӷų��������������䣻���������������1molCl2�����շų�$\frac{1}{3}$molN2��$\frac{1}{3}$molCO2����Ĥ������Һ�������仯���m��-��m�ң�=$\frac{1}{3}$��28+44��+2��1=26g��
�ʴ�Ϊ��26��
���� ���⿼�黯ѧ��Դ���͵�ء����ԭ����֪ʶ�㣬��ȷ�����缫�Ϸ����ķ�Ӧ�ǽⱾ��ؼ����ѵ��ǵ缫��Ӧʽ����д���йؼ��㣬��д�缫��ӦʽҪ��ϵ������Һ�������д��ע�⣨3�����������������ķ�Ӧ��Ϊ�״��㣮

�ش��������⣺
��1�����иĽ����Ż���ˮ�ۺ����ù��յ�������������е��Ǣڢۢܣ�����ţ���
���û�������ȡ��ˮ ����߲��ֲ�Ʒ������
���Ż���ȡ��Ʒ��Ʒ�� �ܸĽ��ء��塢þ����ȡ����
��2�����á���������������Ũ��ˮ�д���Br2�����ô������գ������������Ҫ��Ӧ��Br2+Na2CO3+H2O NaBr+NaBrO3+NaHCO3������1mol Br2ʱ��ת�Ƶĵ�����Ϊ$\frac{5}{3}$mol��
��3����ˮ��þ��һ�ι���������ͼ��

Ũ��ˮ����Ҫ�ɷ����£�
| ���� | Na+ | Mg2+ | Cl- | SO42- |
| Ũ��/g/L | 63.7 | 28.8 | 144.6 | 46.4 |
��4������ʯī���������������������ڵ��Ȼ�þ��������Ӧ�Ļ�ѧ����ʽΪMgCl2�����ڣ�$\frac{\underline{\;ͨ��\;}}{\;}$Mg+Cl2�������ʱ����������ˮ���ڻ���ɲ�Ʒþ�����ģ�д���йط�Ӧ�Ļ�ѧ����ʽMg+2H2O$\frac{\underline{\;ͨ��\;}}{\;}$Mg��OH��2+H2����
| A�� | ������Ϊ17��������Ϊ20����ԭ�ӣ�${\;}_{17}^{20}$Cl | |
| B�� | �����ӣ�Cl-���Ľṹʾ��ͼ��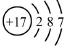 | |
| C�� | �ȷ��ӵĵ���ʽ��${\;}_{•}^{•}$$\underset{\stackrel{••}{Cl}}{••}$${\;}_{•}^{•}$$\underset{\stackrel{••}{Cl}}{••}$${\;}_{•}^{•}$ | |
| D�� | ����ϩ���ӵĽṹ��ʽ��H3C-CH2Cl |
| ���� | ��ȩ | ���� | ���� | �Ҷ��� | ˮ |
| �е� | 20.8�� | 117.9�� | 290�� | 197.2�� | 100�� |
��1���Թ�A����60�桫80��ʱ��������Ҫ��Ӧ�Ļ�ѧ����ʽΪ��ע����Ӧ������2CH3CHO+O2$��_{68�桫80��}^{cuo}$2CH3COOH��
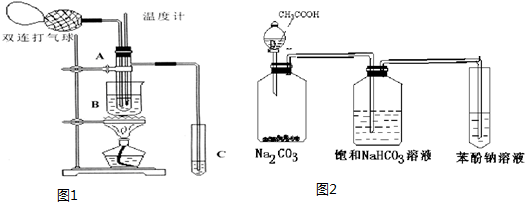
��2����ͼ��ʾ��ʵ��IJ�ͬ�Σ���Ҫ�����¶ȼ����Թ�A�ڵ�λ�ã�
��ʵ�鿪ʼʱ�¶ȼ�ˮ�����λ��Ӧ���Թ�A�ķ�ӦҺ�У�Ŀ���Dz�����Ӧ��Һ���¶ȣ����Թ�A�ڵ���Ҫ��Ӧ��ɺ��¶ȼ�ˮ�����λ��Ӧ�����Թ�A��֧�ܿڴ���Ŀ�����ռ����������֣�
��3���ձ�B�������ǣ���ʹ�Թ�A�ڵķ�ӦҺ�������ȷ�����Ӧ����ʹ���ɵ��������������Թܣ��ձ�B��ʢװ��Һ��������Ҷ�������ͣ�д��һ�ּ��ɣ�������������ң���
��4����������Թ�C���Ƿ��в������ᣬ�������ṩ���Լ�����ֽ�п���ѡ��ad��������ţ���ʵ��������ѡ�����ṩ���Լ�����ֽ�У�
a��pH��ֽ b����ɫ��ʯ����ֽ c����ɫ�Ĵ���Ǧ��ֽ d��̼�����Ʒ�ĩ��
��5��ʵ���ҿ���������װ����ͼ2��̽�����ᡢ���ӡ�̼�������ǿ����
�ٱ���̼��������Һ�������dz�ȥ���ᣮ
�ڱ�������Һ��Ӧ�۲쵽��Һ����ǣ��йصĻ�ѧ����ʽC6H5ONa+CO2+H2O��C6H5OH��+NaHCO3��
| ��ʼŨ�� | �� | �� | �� |
| c��H2��/mol/L | 0.010 | 0.020 | 0.020 |
| c��CO2��/mol/L | 0.010 | 0.010 | 0.020 |
| A�� | ��Ӧ��ʼʱ����Ӧ���ʣ��ף��ң��� | |
| B�� | ƽ��ʱ�����кͱ���H2��ת���ʾ���60% | |
| C�� | ƽ��ʱ��c��CO2����=2c��CO2���� | |
| D�� | ƽ��ʱ������CO2�������������60% |
| A�� | ��������ƽ����11.7g�Ȼ��ƾ��� | |
| B�� | �ü�ʽ�ζ�����ȡ25.00mL���������Һ | |
| C�� | �����ô����������������ơ�̼���� | |
| D�� | �ⶨ��Һ��pHʱ���ýྻ������IJ�����պȡ��Һ������������ˮ��ʪ����pH��ֽ�ϣ��������ɫ���Ƚ� | |
| E�� | ������ˮ��pH��ֽ���Ϳ��Լ���pH��ȵ�H2SO4��CH2COOH��Һ |