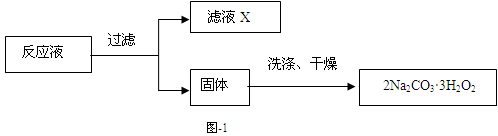
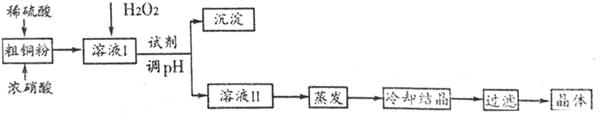
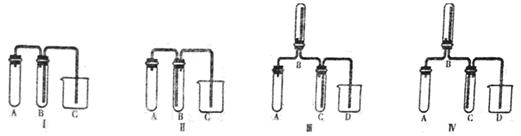
��Ŀ����
���Ȼ���(SCl2)�۵�-78�棬�е�59�棬�ܶ�1��638g��mL����ˮ�ֽ⣬���Ȼ����������������ÿ�������Ҫ�����Լ���������(SOCl2)����������������ϳɶ��Ȼ����ʵ��װ�á�
�Իش��������⣺

��1��װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2��װ��B��CӦʢ�ŵ�ҩƷ�ֱ��� �� ��
��3��ʵ�鿪ʼǰ����D�з�һ��������ۣ�����ʹ���ۻ���Ȼ��ת����ҡ����ƿʹ��������ƿ�ڱ��γ�һ������棬��������Ŀ���� ��
��4��ʵ��ʱ��Dװ���������50��59�森��ò��õĴ�ʩ�� ����η�ֹE��Һ��ӷ��� ��
��5��Fװ���и��������ʢ������ �������� ��
��6���ɶ��Ȼ�����SO3���������������ȵĻ�ѧ����ʽΪ ��
�Իش��������⣺

��1��װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2��װ��B��CӦʢ�ŵ�ҩƷ�ֱ��� �� ��
��3��ʵ�鿪ʼǰ����D�з�һ��������ۣ�����ʹ���ۻ���Ȼ��ת����ҡ����ƿʹ��������ƿ�ڱ��γ�һ������棬��������Ŀ���� ��
��4��ʵ��ʱ��Dװ���������50��59�森��ò��õĴ�ʩ�� ����η�ֹE��Һ��ӷ��� ��
��5��Fװ���и��������ʢ������ �������� ��
��6���ɶ��Ȼ�����SO3���������������ȵĻ�ѧ����ʽΪ ��
��1��MnO2+4HCl(Ũ)
 MnCl2+Cl2��+2H2O (2��)
MnCl2+Cl2��+2H2O (2��)��2������ʳ��ˮ(��ˮ) (1��) Ũ���� (1��)
��3������Ӧ�Ӵ��� (1��)
��4��ˮԡ���ȣ��¶ȼƿ����¶� (2��) ����ƿ���˱�ˮ����ȴ (1��)
��5����ʯ��(���̬�������ƺ���ʯ��)��(1��)��ֹ������ˮ��������E�в����ղ�����������(1��)
��6��SCl2 +SO3 = SOCl2 +SO2 (2��)
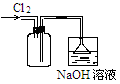
�����������1��װ��AΪ��ȡCl2��װ�ã�ʵ������MnO2��Ũ������ȡCl2��
��2����ȡ�������к�HCl��H2O���ʣ��ֱ��ñ���ʳ��ˮ��Ũ�����ȥ��
��3������۱�ɱ��㣬���������������ӿ췴Ӧ���ʡ�
��4������100��ļ���һ�����ˮԡ���ȣ�ʹ���¶ȼ��ܸ��õĿ��Ʒ�Ӧ�¶ȣ��ڱ�ˮ����ȴ�ɽ����¶ȣ���ֹ������Ļӷ���
��5��β���к���δ��Ӧ�����������Ȼ�����ˮ�ֽ⣬���Ը��������ʢ���������������ͷ�ֹ�������е�ˮ�ֽ��룬��ѡ�ü�ʯ��(���̬�������ƺ���ʯ��)��
��6��������Ŀ�������Եó�SCl2��SO3Ϊ��Ӧ�SOCl2 Ϊ�������������ԭ��Ӧ���ɿɵó���һ����ΪSO2��Ȼ���д����ѧ����ʽ��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ