题目内容
【题目】常温下,向0.1mol·L1二元弱酸H2A溶液中加入氢氧化钾固体改变溶液的pH,溶液中的H2A、HA、A2的物质的量分数![]() (X)随pH的变化如图所示[已知
(X)随pH的变化如图所示[已知![]() (X) =
(X) =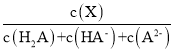 ]。下列叙述错误的是
]。下列叙述错误的是
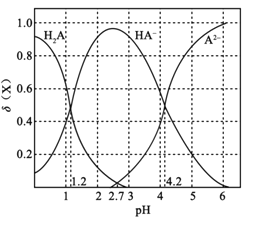
A.pH=1.2时,c(K+)+c(H+)=c(H2A)+c(OH)
B.常温下,H2A的电离平衡常数Ka2=1042
C.pH=2.7时,c(HA)>c(H2A)=c(A2)
D.KHA溶液中离子浓度为c(K+)>c(HA)>c(OH)>c(H2A)>c(H+)>c(A2)
【答案】D
【解析】
A项、由图像可知,pH=1.2时,二元弱酸H2A溶液与氢氧化钾固体反应得到H2A 和KHA混合溶液,溶液中c(H2A)=c(HA)、c(A2)=0,由溶液中的电荷守恒关系c(K+)+c(H+)=c(OH)+c(HA)+2 c(A2)可得c(K+)+c(H+)=c(H2A)+c(OH),故A正确;
B项、由图像可知,pH=4.2时,二元弱酸H2A溶液与氢氧化钾固体反应得到K2A 和KHA混合溶液,溶液中c(H+)=104.2 mol/L,c(HA)=c(A2),则H2A的电离平衡常数Ka2= 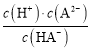 = c(H+)=104.2,故B正确;
= c(H+)=104.2,故B正确;
C项、由图像可知,pH=2.7时,二元弱酸H2A溶液与氢氧化钾固体反应得到H2A 、K2A 和KHA混合溶液,溶液中c(H2A)=c(A2),由纵坐标数据可知c(HA)>c(H2A)=c(A2),故C正确;
D项、由图像可知,KHA溶液显酸性,溶液中c(H+)>c(OH),说明溶液中HA的电离大于HA的水解,则溶液中c(A2)>c(H2A),故D错误;
故选D。

【题目】在3个体积均为4.0 L的恒容密闭容器中,反应 CO2(g)+C(s)![]() 2CO(g) ΔH > 0,分别在一定温度下达到化学平衡状态。下列说法正确的是
2CO(g) ΔH > 0,分别在一定温度下达到化学平衡状态。下列说法正确的是
容器 | 温度/K | 起始时物质的量/mol | 平衡时物质的量/mol | ||
n(CO2) | n(C) | n(CO) | n(CO) | ||
I | 977 | 0.56 | 1.12 | 0 | 0.8 |
II | 977 | 1.12 | 1.12 | 0 | x |
III | 1250 | 0 | 0 | 1.12 | y |
A.977 K,该反应的化学平衡常数值为4
B.达到平衡时,向容器I中增加C的量,平衡正向移动
C.达到平衡时,容器I中CO2的转化率比容器II中的大
D.达到平衡时,容器III中的CO的转化率大于28.6%