��Ŀ����
ijУ��ѧ�о���ѧϰС����������˽�������ݣ�
�Ҷ���(HOOC-COOH���ɼ�дΪH2C2O4)�׳Ʋ��ᣬ������ˮ�����ڶ�Ԫ��ǿ��(Ϊ�������)��������ǿ��̼�ᣬ���۵�Ϊ101.5�棬��157��������Ϊ̽������IJ��ֻ�ѧ���ʣ�����������ʵ�飺
(1)��ʢ��1 mL����NaHCO3��Һ���Թ��м��������Ҷ�����Һ���۲쵽����ɫ���ݲ������÷�Ӧ�����ӷ���ʽΪ__________________________________________��
(2)��ʢ���Ҷ��ᱥ����Һ���Թ��е��뼸�������ữ��KMnO4��Һ������������Һ���Ϻ�ɫ��ȥ��˵���Ҷ������_____________(������ԡ�������ԭ�ԡ������ԡ�)������ƽ�÷�Ӧ�����ӷ���ʽ��
____ MnO4�C + ____ H2C2O4 + _____ H+ = _____ Mn2+ + _____ CO2�� + _____ H2O
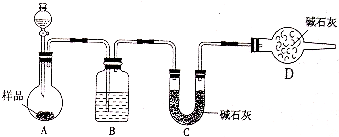
(3)��һ�������Ҷ�������Թ��У�����ͼ��ʾװ�ý���ʵ��(�г�װ��δ���)��

ʵ�鷢�֣�װ��C��G�г���ʯ��ˮ����ǣ�B��CuSO4��ĩ������F��CuO��ĩ��졣�ݴ˻ش�
����װ���У�D��������__________________��
�Ҷ���ֽ�Ļ�ѧ����ʽΪ_____________________________________��
(4)��С��ͬѧ��2.52 g���ᾧ��(H2C2O4��2H2O)���뵽100 mL 0.2 mol/L��NaOH��Һ�г�ַ�Ӧ����÷�Ӧ����Һ�����ԣ���ԭ����__________________��(�����ּ���)
(5)������Һ�и����ӵ�Ũ���ɴ�С��˳��Ϊ��_____________________________���������ӷ��ű�ʾ��
(1)HCO3�C + H2C2O4 = HC2O4�C+ CO2��+ H2O(2��)
(2) ��ԭ��(2��) 2 5 6 2 10 8��2��)
(3) ��ȥ��������е�CO2(2��) H2C2O4![]() H2O+CO��+CO2�� (2��)
H2O+CO��+CO2�� (2��)
(4)��Ӧ������ҺΪNaHC2O4��Һ������HC2O4�C�ĵ���̶ȱ�ˮ��̶ȴ�����Һ��c(H+) > c(OH�C)��������Һ������(2��)
(5)Na+>HC2O4->H+> C2O42->OH-(3��)
����:
��


 ijУ��ѧ�о���ѧϰС���������ʵ�鷽�����ⶨ���ü��õ�С�մ���Ʒ�д��������������
ijУ��ѧ�о���ѧϰС���������ʵ�鷽�����ⶨ���ü��õ�С�մ���Ʒ�д��������������