��Ŀ����
����Ŀ��HecK��Ӧ��ż����Ӧ��һ�֣����磺![]() +
+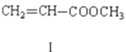
![]()
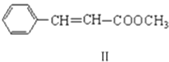 ��������������������;���ϳɣ���(����ʽC3H6)
��������������������;���ϳɣ���(����ʽC3H6)![]() ��
��![]() ��
��![]() I
I
(1)���������ķ���ʽΪ_______��1mol������������________molH2�����ӳɷ�Ӧ��
(2)±����CH3CHBrCH3������ȥ��Ӧ�������ɻ�����������Ӧ�Ļ�ѧ����Ϊ_______(ע������)��
(3)�����������м�����֧����ͬϵ�����ķ���ʽΪC4H6O���������Ľṹ��ʽΪ____��
(4)������������CH3CH2OH����������Ӧ���ɻ�������������������һ�������¿��Է����Ӿۣ�����Ӿ۲���Ľṹ��ʽΪ_____��
(5)����������һ��ͬ���칹�����ܷ���������Ӧ���Һ˴Ź�������ֻ������壬�����֮��Ϊ1:2:2�����Ľṹ��ʽΪ____��
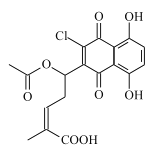
(6)һ�������£�![]() ��
��![]() Ҳ���Է������Ʒ�Ӧ�ٵķ�Ӧ�����л�����Ľṹ��ʽΪ_____��
Ҳ���Է������Ʒ�Ӧ�ٵķ�Ӧ�����л�����Ľṹ��ʽΪ_____��
���𰸡�C10H10O2 4 CH3CHBrCH3+NaOH![]() CH2=CHCH3+NaBr+H2O CH3CH=CHCHO
CH2=CHCH3+NaBr+H2O CH3CH=CHCHO ![]()
![]()
![]()
��������
ͨ�������Ľṹ��ʽ�ķ�����֪��C3H6Ϊ��ϩ����CH3CH=CH2���ӷ�Ӧ������֪����������ȩ���������ķ�Ӧ����֪����ΪCH2=CHCHO����ΪCH2=CHCOOH���ݴ˷������
(1)ͨ�����Ľṹ��ʽ���ɵ������ʽΪC10H10O2�����������������ӳɣ�ֻ�б�����̼̼˫�������������ӳɣ�����1mol������������4mol���������ӳɷ�Ӧ���ʴ�Ϊ��C10H10O2��4��
(2)±������ȥ��Ӧ��������NaOH���Ҵ���Һ���ȣ������䷴Ӧ����ʽΪCH3CHBrCH3+NaOH![]() CH2=CHCH3+NaBr+H2O���ʴ�Ϊ��CH3CHBrCH3+NaOH
CH2=CHCH3+NaBr+H2O���ʴ�Ϊ��CH3CHBrCH3+NaOH![]() CH2=CHCH3+NaBr+H2O��
CH2=CHCH3+NaBr+H2O��
(3)��������ΪCH2=CHCHO���京�м�����֧����ͬϵ����ֻ����CH3CH=CHCHO���ʴ�Ϊ��CH3CH=CHCHO��
(4))��ΪCH2=CHCOOH������CH3CH2OH����������Ӧ���ɻ�����������ΪCH2=CHCOOCH2CH3���䷢���Ӿ۷�Ӧʱ����̼̼˫���ļӾۣ�����Ϊ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(5)���������Ľṹ��ʽΪ![]() ����ͬ���칹�����ܷ���������Ӧ������ȩ�����Һ˴Ź�������ֻ������壬�����֮��Ϊ1:2:2�����������ֵ�Ч���Ҹ�����Ϊ1:2:2�������������������Ľṹ��ʽΪ
����ͬ���칹�����ܷ���������Ӧ������ȩ�����Һ˴Ź�������ֻ������壬�����֮��Ϊ1:2:2�����������ֵ�Ч���Ҹ�����Ϊ1:2:2�������������������Ľṹ��ʽΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(6)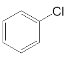 ��
��![]() Ҳ���Է������Ʒ�Ӧ�ٵķ�Ӧ��
Ҳ���Է������Ʒ�Ӧ�ٵķ�Ӧ�� ��Clԭ�ӱ�
��Clԭ�ӱ�![]() ȡ�������л�����Ľṹ��ʽΪ
ȡ�������л�����Ľṹ��ʽΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��

 ������������ϵ�д�
������������ϵ�д�����Ŀ��(1)���������õ������£�NH4+����������Ӧ��������NO3-��������Ӧ�������仯ʾ��ͼ���£�
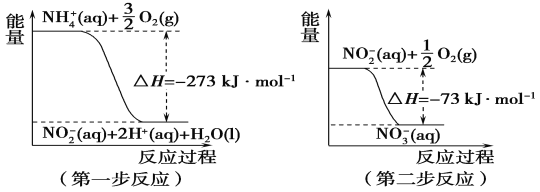
�ٵ�һ����Ӧ��________(��������������������)��Ӧ��
��1 mol NH4+(aq)ȫ��������NO3-(aq)���Ȼ�ѧ����ʽ��__________________��
(2)��298 K��101 kPaʱ����֪��2H2O(g)=O2(g)+2H2(g)����H1��
Cl2(g)+H2(g)=2HCl(g)�� ��H2��
2Cl2(g)+2H2O(g)=4HCl(g)+O2(g)����H3��
����H3����H1����H2֮��Ĺ�ϵ��ȷ����___________��
A.��H3=��H1+2��H2 B.��H3=��H1+��H2
C.��H3=��H1-2��H2 D.��H3=��H1-��H2
(3)��֪���ױȰ����ȶ�����ӦP4(���ף�s)+5O2(g)=2P2O5(s)����H1��P(���ף�s)��5O2(g)=2P2O5(s)����H2����H1����H2�Ĺ�ϵ����H1_________��H2(����>������<������=��)��
(4)��֪H2(g)��Br2(l)=2HBr(g)����H=-72 kJ/mol������1 mol Br2(l)��Ҫ���յ�����Ϊ30 kJ����������������±���
���� | H2(g) | Br2(g) | HBr(g) |
1 mol�����еĻ�ѧ������ʱ��Ҫ���յ�����(kJ) | 436 | 200 | a |
�����a=________��