��Ŀ����
17������������Ϊ98%���ܶ�Ϊ1.84g•cm-3��Ũ��������l mol•L-1 ϡ����100mL������������¸�����������Ͳ��ȡ5.4mLŨ����
��ϴ���ձ��Ͳ�����2-3�Σ���ϴ��Һת������ƿ��
�۽�ϡ�͡���ȴ�������ת������ƿ��
�ܽ�Ũ���ᵹ��ʢ��ˮ���ձ���ϡ�͡���ȴ
�ݼ�ˮ��Һ��ӽ��̶���1��2cm�������ݣ�ҡ��
���������գ�
��1���ڢٲ�������Ӧ����Ͳ��ȡ5.4mLŨ���
��2����ʵ���õ��Ļ������������ձ�����Ͳ������������ȱ�ٵ�������100mL����ƿ�ͽ�ͷ�ιܣ�
��3����ȷ�IJ���˳���ǣ��������д���٢ܢۢݢڣ�
��4��ʵ���У�������Щ�����ɵ��������Ƶ�����Ũ�Ƚ�ƫ��BC��
A������ƿ�ڱڸ���ˮ���δ���и��ﴦ�� B�����ǽ�ϴ��Һ��������ƿ
C������ʱ�����ӿ̶��� D�����ݺ�ҡ�ȣ�Һ����ڿ̶��ߣ�
���� ��1������c=$\frac{1000�Ѧ�}{M}$����Ũ��������ʵ���Ũ�ȣ��ٸ�����Һϡ��ǰ�����ʵ��������������Ũ����������
��2������������Һ��ʵ���������ѡ������������
��3������������Һ��ʵ��������̽��в���˳�������
��4���������������ʵ����ʵ��������Һ�������Ӱ�죬����c=$\frac{n}{V}$�����жϣ�
��� �⣺��1��ŨH2SO4�����ʵ���Ũ��c=$\frac{1000��1.84��98%}{98}$mol/L=18.4mol/L������ϡ�Ͷ��ɣ�ϡ��ǰ�����ʵ����ʵ������䣬������Ũ������������Ũ��������ΪxmL������xmL��18.4mol/L=100mL��1mol/L����ã�x��5.4��
�ʴ�Ϊ��5.4��
��2�����������м��㡢��ȡ��ϡ�͡���Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ���������Ͳ��ȡ���õ���ͷ�ιܣ�Ũ���ᣬ���ձ���ϡ�ͣ��ò��������裬��ȴ��ת�Ƶ�100mL����ƿ�У����ò�����������ϴ��2-3�Σ���ϴ��Һת�Ƶ�����ƿ�У���ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ�
���ṩ��������֪����Ҫ�����У�100mL����ƿ����ͷ�ιܣ�
�ʴ�Ϊ��100mL����ƿ����ͷ�ιܣ�
��3���ɣ�2���в��������֪��ȷ�IJ���˳���Ǣ٢ܢۢݢڣ��ʴ�Ϊ���٢ܢۢݢڣ�
��4��A������ƿ�ڱڸ���ˮ���δ���и��ﴦ������Ũ����Ӱ�죬��A����
B�����ǽ�ϴ��Һ��������ƿ���ᵼ�����ʵ���ʧ����Ũ��ƫ�ͣ���B��ȷ��
C������ʱ�����ӿ̶��ߣ�����Һ���ƫ��Ũ��ƫ�ͣ���C��ȷ��
D�����ݺ�ҡ�ȣ�Һ����ڿ̶����������ģ���Ũ����Ӱ�죬��D����
��ѡBC��
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƹ����еļ���������������ڻ�������Ŀ���ѶȲ���

 ��У����ϵ�д�
��У����ϵ�д�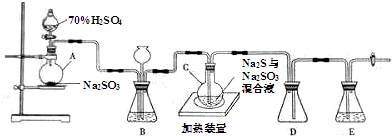
��ƿC�з�����Ӧ���£�
��Na2S+H2O+SO2�TNa2SO3+H2S
��2H2S+SO2�T3S+2H2O
��S+Na2SO3$\frac{\underline{\;\;��\;\;}}{\;}$Na2S2O3
��1��������װ��ɺر����˻�������װ��B�еij���©����ע��Һ�����γ�һ��Һ������Һ��Һ�治�½���������װ�����������ã�
��2��Ϊ��߲�Ʒ���ȣ�Ӧʹ��ƿC��Na2SO3��Na2Sǡ����ȫ��Ӧ������ƿC��Na2SO3��Na2S���ʵ���֮��Ϊ1�s2��
��3��װ��B������֮һ�ǹ۲�SO2���������ʣ����е�Һ�����ѡ��c��
a������NaOH��Һ b������Na2SO3��Һ
c������NaHSO3��Һ d�� ���������Һ
��4����Ӧ��ֹ����ƿC�е���Һ������Ũ����������Na2S2O3•5H2O�����п��ܺ���Na2SO3��Na2SO4�����ʣ���֪Na2S2O3•5H2O�����ֽ⣺S2O${\;}_{3}^{2-}$+2H+�TS��+SO2��+H2O�����������Լ���ϡ���ᡢϡ���ᡢϡ���ᡢBaCl2��Һ��AgNO3��Һ�����ʵ�飬����Ʒ���Ƿ����Na2SO4����Ҫ˵��ʵ�����������ͽ��ۣ�ȡ��������ˮ�����Һ����������ϡ�����������ݲ��������ϲ���Һ�е���BaCl2��Һ�����г������ɣ������Na2SO4��
��5��Ϊ�˲ⶨij������Ʒ�ijɷ֣���ȡ����������ͬ�ĸ���Ʒ���ֱ������ͬŨ�ȵ������� Һ25mL����ַ�Ӧ���˳�������Һʹ���ɵ�SO2ȫ���ݳ���
����й�ʵ���������£���״������
| ��һ�� | �ڶ��� | ������ | |
| ��Ʒ������/g | 12.60 | 18.90 | 28.00 |
| ������������/L | 1.12 | 1.68 | 2.24 |
| A�� | 3�� | B�� | 4�� | C�� | 5�� | D�� | 6�� |
| A�� | ��Ӧֹͣ�� | B�� | ����Ӧ�������淴Ӧ���ʾ�Ϊ�� | ||
| C�� | ��Ӧ���������Ũ����� | D�� | ����Ӧ�������淴Ӧ������� |

 ���з�Ӧ��CO2��g��+H2��g��?CO��g��+H2O��g����H��0����ͼ��ʾ��Ӧ����t1ʱ�̴ﵽƽ�⣬��t2ʱ����ı�ij�������������仯���������ͼ��t2ʱ�̷����ı������������B��
���з�Ӧ��CO2��g��+H2��g��?CO��g��+H2O��g����H��0����ͼ��ʾ��Ӧ����t1ʱ�̴ﵽƽ�⣬��t2ʱ����ı�ij�������������仯���������ͼ��t2ʱ�̷����ı������������B��