��Ŀ����
����A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ��������������������EԪ�صĵ��������Ũ���������У����涼�������ܵ�����Ĥ��DԪ��ԭ�Ӻ���M���Ӳ���K���Ӳ��ϵĵ�������ȣ�A�ɷֱ���B��F�γ�A2B��A2B2��AF�ȹ��ۻ����C�ɷֱ���B��F�γ�C2B��C2B2��CF�����ӻ����
��ش��������⣺
��1����������Ԫ���У���������ǿ���ǣ���Ԫ�ط��ţ�
 ��
��
��2������Ԫ���γɵļ������У������������ȵ��ǣ�����ʵ�����ӷ��ţ�
��3��C2B2�ĵ���ʽΪ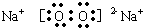
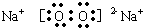 ���������д��ڵĻ�ѧ����
���������д��ڵĻ�ѧ����
��4��A2B��C2B2��Ӧ�Ļ�ѧ����ʽ��
��ش��������⣺
��1����������Ԫ���У���������ǿ���ǣ���Ԫ�ط��ţ�
Na
Na
��Fԭ�ӵĽṹʾ��ͼΪ

��2������Ԫ���γɵļ������У������������ȵ��ǣ�����ʵ�����ӷ��ţ�
O2-��Na+��Mg2+��Al3+
O2-��Na+��Mg2+��Al3+
����3��C2B2�ĵ���ʽΪ
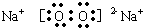
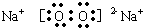
���Ӽ����Ǽ��Լ����ۼ���
���Ӽ����Ǽ��Լ����ۼ���
����4��A2B��C2B2��Ӧ�Ļ�ѧ����ʽ��
2H2O+2Na2O2=4NaOH+O2��
2H2O+2Na2O2=4NaOH+O2��
����������ͨ��������Ϣ�ƶ�Ԫ�أ�����������EԪ�صĵ��������Ũ���������У����涼�������ܵ�����Ĥ��E��Al����DԪ��ԭ�Ӻ���M���Ӳ���K���Ӳ��ϵĵ�������ȵ�D��Mg��A�ɷֱ���B��F�γ�A2B��A2B2��AF�ȹ��ۻ����A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ������������������A�ɷֱ���B��F�γ�H2O��H2O2��HCl������A��B��F�ֱ���H��O��ClԪ�أ�C�ɷֱ���B��F�γ�C2B��C2B2��CF�����ӻ������C2B��C2B2��CF��C�Ļ��ϼ���+1�ۣ�ԭ������С��D������C����Ԫ�أ���A��B��C��D��E��F�ֱ���H��O��Na��Mg��Al��Cl��Ȼ���ٸ������������ڱ��е�λ�ã��Ʋ�Ԫ�ص����ʣ�
����⣺��������EԪ�صĵ��������Ũ���������У����涼�������ܵ�����Ĥ��E��Al����DԪ��ԭ�Ӻ���M���Ӳ���K���Ӳ��ϵĵ�������ȵ�D��Mg��A�ɷֱ���B��F�γ�A2B��A2B2��AF�ȹ��ۻ����A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ������������������A�ɷֱ���B��F�γ�H2O��H2O2��HCl������A��B��F�ֱ���H��O��ClԪ�أ�C�ɷֱ���B��F�γ�C2B��C2B2��CF�����ӻ������C2B��C2B2��CF��C�Ļ��ϼ���+1�ۣ�ԭ������С��D������C����Ԫ�أ���A��B��C��D��E��F�ֱ���H��O��Na��Mg��Al��Cl��
��1������ԭ��������֪��ͬһ���ڣ�����������ԭ���������������С�����Խ�������ǿ�����ƣ�F����Ԫ�أ���ԭ�Ӻ�����17�����ӣ�������17�����ӣ�
�ʴ�Ϊ��Na�� ��
��
��2��H��O��Na��Mg��Al��Cl�γɵļ����ӷֱ��ǣ�H+��O2-��Na+��Mg2+��Al3+��Cl-�������������ȵ������ǣ�O2-��Na+��Mg2+��Al3+���ʴ�Ϊ��O2-��Na+��Mg2+��Al3+��
��3��C2B2�ǹ������ƣ���Ԫ�غ���Ԫ��֮���γ����Ӽ�����ԭ�Ӻ���ԭ��֮���γɷǼ��Թ��ۼ����ʴ�Ϊ��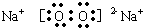 �����Ӽ����Ǽ��Թ��ۼ���
�����Ӽ����Ǽ��Թ��ۼ���
��4��A2B��ˮ��C2B2�ǹ������ƣ�ˮ�������Ʒ�Ӧ�����������ƺ��������ʴ�Ϊ��2H2O+2Na2O2=4NaOH+O2����
��1������ԭ��������֪��ͬһ���ڣ�����������ԭ���������������С�����Խ�������ǿ�����ƣ�F����Ԫ�أ���ԭ�Ӻ�����17�����ӣ�������17�����ӣ�
�ʴ�Ϊ��Na��
 ��
����2��H��O��Na��Mg��Al��Cl�γɵļ����ӷֱ��ǣ�H+��O2-��Na+��Mg2+��Al3+��Cl-�������������ȵ������ǣ�O2-��Na+��Mg2+��Al3+���ʴ�Ϊ��O2-��Na+��Mg2+��Al3+��
��3��C2B2�ǹ������ƣ���Ԫ�غ���Ԫ��֮���γ����Ӽ�����ԭ�Ӻ���ԭ��֮���γɷǼ��Թ��ۼ����ʴ�Ϊ��
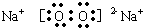 �����Ӽ����Ǽ��Թ��ۼ���
�����Ӽ����Ǽ��Թ��ۼ�����4��A2B��ˮ��C2B2�ǹ������ƣ�ˮ�������Ʒ�Ӧ�����������ƺ��������ʴ�Ϊ��2H2O+2Na2O2=4NaOH+O2����
������������ɣ���ͨ��������Ϣ�ƶ�Ԫ�أ�Ȼ���ٸ������������ڱ��е�λ�ã��Ʋ�Ԫ�ص����ʣ�

��ϰ��ϵ�д�
�����Ŀ
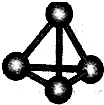 ����A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ��������������D��E���⻯����ӹ��Ͷ���V�ͣ�A��B������������֮����C��������������ȣ�A�ֱܷ���B��C��D�γɵ���������ȵķ��ӣ���A��D���γɵĻ���������¾�ΪҺ̬��
����A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ��������������D��E���⻯����ӹ��Ͷ���V�ͣ�A��B������������֮����C��������������ȣ�A�ֱܷ���B��C��D�γɵ���������ȵķ��ӣ���A��D���γɵĻ���������¾�ΪҺ̬��






