��Ŀ����
����Ŀ��������пհס�
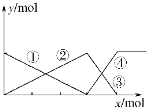
��1��������ֵ��������ʾ���͵�������������������ı���̶���С����������ֵ�궨Ϊ100����ͼ������������ģ�ͣ����������ϵͳ����Ϊ_________________��
��2��д�����з�Ӧ�Ļ�ѧ����ʽ�ͷ�Ӧ����
��2���������NaOH��Һ��________________________��
�ڱ��Ӻͱ�����ˮ��____________________________��
��3���л���A����ͨ����ͬ�ķ�Ӧ�õ�B��C��
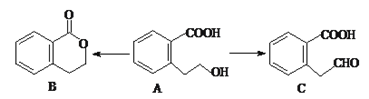
��A�ķ���ʽΪ___________________��C�ĺ�������������Ϊ___________________��
��A��ȡB���л���Ӧ����Ϊ________________��
��A��ȡC�Ļ�ѧ����ʽΪ��_____________________________________________��
��A������ȥ��Ӧ�����Ľṹ��ʽΪ___________________��
���𰸡� 2��2��4-�������� CH3CHBrCH3+NaOH![]() CH3CHOHCH3+NaBr��ȡ����ˮ�⣩��Ӧ
CH3CHOHCH3+NaBr��ȡ����ˮ�⣩��Ӧ 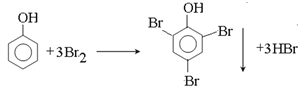 ��ȡ����Ӧ C9H10O3 �Ȼ���ȩ�� ȡ����Ӧ����������Ӧ��
��ȡ����Ӧ C9H10O3 �Ȼ���ȩ�� ȡ����Ӧ����������Ӧ�� ![]()
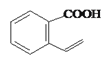 ��
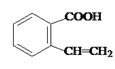
��
����������������1������������Ľṹ��ʽ������
��2����±�������������Ƶ�ˮ��Һ�з���ˮ�ⷴӦ��
�ڱ��Ӻ�Ũ��ˮ����ȡ����Ӧ��
��3���ٸ���A�ļ���ʽ��д����ʽ�����ݽṹ��ʽ�жϹ����ţ�
�ڸ���A��B�����й����ŵı仯�жϷ�Ӧ���ͣ�
�۸����ǻ���������������ȩ����д��
�ܸ����ǻ�������ȥ��Ӧ����̼̼˫���жϡ�
��⣺��1����������������ģ�Ϳ�֪��������5��̼ԭ�ӣ�3������֧�������������ϵͳ����Ϊ2��2��4-�������顣
��2����2���������NaOH��Һ����ˮ�ⷴӦ����2���������廯�ƣ�����ʽΪCH3CHBrCH3+NaOH![]() CH3CHOHCH3+NaBr��
CH3CHOHCH3+NaBr��
�ڱ��Ӻͱ�����ˮ����ȡ����Ӧ�������屽�Ӻ��廯�⣬����ʽΪ ��
��
��3���ٸ���A�Ľṹ��ʽ��֪A�ķ���ʽΪC9H10O3��C�ĺ�������������Ϊ�Ȼ���ȩ����
��A��ȡB�ķ�Ӧ���Ȼ����ǻ�����������Ӧ������������Ӧ����Ϊȡ����Ӧ��
��A��ȡC���ǻ��Ĵ���������Ӧ�Ļ�ѧ����ʽΪ![]() ��
��
��A�����к����ǻ���������ȥ��Ӧ�����Ľṹ��ʽΪ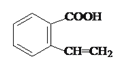 ��
��

 �ο�ͨ�γ̱�˼ά����������ѵ��ϵ�д�
�ο�ͨ�γ̱�˼ά����������ѵ��ϵ�д�