��Ŀ����
7��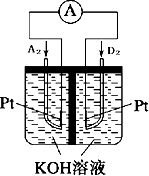 A��B��C��D��E��F��G��Ϊ������Ԫ�أ�ԭ���������ε�����AԪ��ԭ�Ӻ��������ӣ�BԪ��ԭ�������������Ǵ�����������2����D�ǵؿ��к�������Ԫ�أ�E�Ƕ������н�������ǿ��Ԫ�أ�F��Gλ�����ڣ�G��ͬ����Ԫ����ԭ�Ӱ뾶��С��Ԫ�أ����û�ѧ����ش�
A��B��C��D��E��F��G��Ϊ������Ԫ�أ�ԭ���������ε�����AԪ��ԭ�Ӻ��������ӣ�BԪ��ԭ�������������Ǵ�����������2����D�ǵؿ��к�������Ԫ�أ�E�Ƕ������н�������ǿ��Ԫ�أ�F��Gλ�����ڣ�G��ͬ����Ԫ����ԭ�Ӱ뾶��С��Ԫ�أ����û�ѧ����ش���1���ƶ�BԪ����Ԫ�����ڱ��е�λ�õڶ����ڢ�A�壬д��C�ĵ��ʵĵ���ʽ
 ��
����2��A��D�γɵ�18���ӵĻ�������FD2��������һ��ǿ�ᣬ�仯ѧ����ʽΪH2O2+SO2�TH2SO4��
��3��E��F��G����Ԫ�����γɵļ����ӣ������Ӱ뾶�ɴ�С��˳����S2-��Cl-��Na+���� �������ӷ��ű�ʾ����4���õ���ʽ��ʾ������E2F���γɹ���
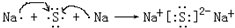 ��
����5��ͼΪij���ͷ���װ��ʾ��ͼ���为���缫��ӦΪH2-2e-+2OH-=2H2O��
��6����101kPa��25���£�16g Һ̬C2A4��D2����ȫȼ����������C2���ų�312kJ��������C2A4��D2��Ӧ���Ȼ�ѧ����ʽΪN2H4��l��+O2��g���TN2��g��+2H2O��l����H=-624KJ•mol-1��
���� A��B��C��D��E��F��Ϊ������Ԫ�أ�ԭ���������ε�����AԪ��ԭ�Ӻ��������ӣ���AΪ��Ԫ�أ�BԪ��ԭ�Ӻ��������������Ǵ�����������2������B��2�����Ӳ㣬�������4�����ӣ���BΪ̼Ԫ�أ�DԪ���ǵؿ��к�������Ԫ�أ���DΪ��Ԫ�أ�ԭ���������ε�������C�ǵ���EԪ���Ƕ�����Ԫ���н�������ǿ��Ԫ�أ���EΪNa��F��G��λ�����ڣ�G��ͬ����Ԫ����ԭ�Ӱ뾶��С��Ԫ�أ�����֪FΪSԪ�ء�GΪClԪ�أ��ݴ˽��
��� �⣺��1��BΪ̼Ԫ�أ�λ�ڵڶ����ڢ�A�壬C�ĵ����ǵ���������ʽΪ�� ���ʴ�Ϊ���ڶ����ڢ�A�壻
���ʴ�Ϊ���ڶ����ڢ�A�壻 ��
��
��2��A��D�γɵ�18���ӵĻ������ǹ������⣬��FD2�����Ƕ����������߷�Ӧ����һ�����ᣬ��Ӧ����ʽΪ��H2O2+SO2�TH2SO4���ʴ�Ϊ��H2O2+SO2�TH2SO4��
��3�����Ӳ���Խ��뾶Խ���Ӳ�����ͬʱ�˵����Խ��뾶ԽС�������ơ���������Ԫ�����γɵļ����ӣ����Ӱ뾶�ɴ�С��˳���� S2-��Cl-��Na+���ʴ�Ϊ��S2-��Cl-��Na+��
��4���������ӻ������γɹ���Ϊ��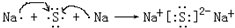 ���ʴ�Ϊ��
���ʴ�Ϊ��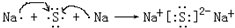 ��
��
��5�������Ƿ���������Ӧ������ʧ���ӣ�ע�������Ǽ���Ե缫��ӦʽΪ��H2-2e-+2OH-=2H2O���ʴ�Ϊ��H2-2e-+2OH-=2H2O��
��6����101kPa��25��ʱ��ʱ����֪0.5molҺ̬��������������Ӧ�����ɵ�����ˮ�������ų�312KJ����������1molҺ̬����ȫ��Ӧ���ɵ�����ˮ�����ų�������Ϊ��312kJ��2=624kJ���÷�Ӧ���Ȼ�ѧ����ʽΪ��N2H4��1��+O2��g��=N2��g��+2H2O��g����H=-624kJ/mol���ʴ�Ϊ��N2H4��l��+O2��g���TN2��g��+2H2O��l����H=-624 KJ•mol-1��
���� ���⿼��ṹ����λ�ù�ϵӦ�ã��漰����ʽ���Ȼ�ѧ����ʽ��������ԭ��Ӧ�ȣ����ض�֪ʶ��������Ǩ��Ӧ���������飬�Ѷ��еȣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | ���ӻ�������һ��ֻ�����Ӽ� | |
| B�� | ���ۻ�������һ��ֻ�����ۼ� | |
| C�� | ���ӻ������в�һ�����н���Ԫ�� | |
| D�� | ���ۼ���һ��ֻ�����ڹ��ۻ������� |
| A�� | �Ҵ���Ũ�����ᷴӦCH3CH2OH+HBr$\stackrel{��}{��}$CH3CH2Br+H2O | |
| B�� | ������������������Һ����CH3CH2Br+NaOH$��_{��}^{ˮ}$ CH3CH2OH+NaBr | |
| C�� | ��������ͨ�������Ķ�����̼2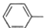 ONa+CO2+H2O��2 ONa+CO2+H2O��2 OH+Na2CO3 OH+Na2CO3 | |
| D�� | ��ȩ������ 2CH3CHO+O2$��_{��}^{����}$2CH3COOH |
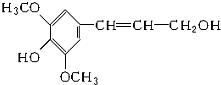 ��ʳ��ά����ͻ���ı������ܣ�������ġ�����Ӫ���ء���ľ������һ�ַ�������ʳ��ά���䵥��֮һ--���Ӵ��ṹ��ʽ��ͼ��ʾ�������йؽ��Ӵ���˵����ȷ���ǣ�������
��ʳ��ά����ͻ���ı������ܣ�������ġ�����Ӫ���ء���ľ������һ�ַ�������ʳ��ά���䵥��֮һ--���Ӵ��ṹ��ʽ��ͼ��ʾ�������йؽ��Ӵ���˵����ȷ���ǣ�������| A�� | ���Ӵ������������ֺ��������� | |
| B�� | ���Ӵ�����������̼ԭ�Ӳ�������ͬһƽ���� | |
| C�� | 1 mol���Ӵ�������Ũ��ˮ��Ӧ���������3 mol Br2 | |
| D�� | ���Ӵ��ܷ����ķ�Ӧ������������ȡ�����ӳ� |
| A�� | �Ȼ��Ƶĵ���ʽ��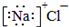 | B�� | �Ȼ���ĵ���ʽ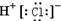 | ||
| C�� | ̼Ԫ��λ�����ڱ��е�2���ڢ�A�� | D�� | H2O�Ľṹʽ��H-O-H |
| A�� | ���ࡢ��֬�������ʾ�Ϊ�߷��ӻ����� | |
| B�� | �����ڶ��ױ���Ϊͬϵ�� | |
| C�� | �ޡ��顢��ë���ϳ���άͳ��Ϊ��ѧ��ά | |
| D�� | �ϳɾ���ϩ��ϳɷ�ȩ��֬�ķ�Ӧ������ͬ |
| A�� | N2O������� | |
| B�� | N2O��CO2�������Ǽ��Լ� | |
| C�� | N2O�ĵ���ʽ�ɱ�ʾ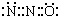 | |
| D�� | N2O��SiO2Ϊ�ȵ����塢�������ƵĽṹ���������� |

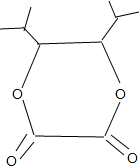 +2H2O��
+2H2O�� ��
��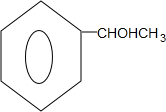 $��_{��}^{Ũ����}$
$��_{��}^{Ũ����}$ +H2O��
+H2O��