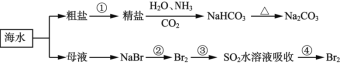
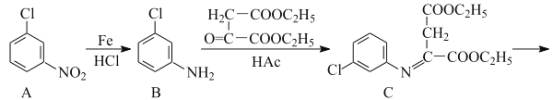
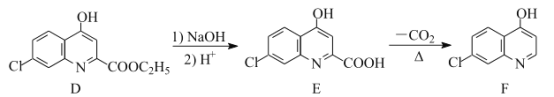
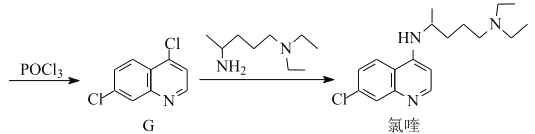
题目内容
【题目】钛被誉为“现代金属”和“战略金属”。
(1)Ti基态核外电子排布式为___。
(2)TiO2与BaCO3一起熔融可制得偏钛酸钡,CO32-的空间构型为___;与CO32-互为等电子体的分子是___。
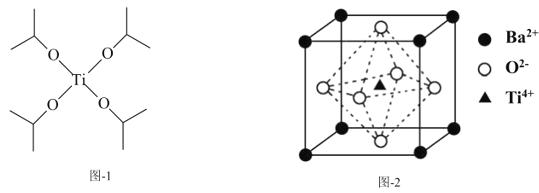
(3)四异丙醇钛(C12H28O4Ti)结构如图-1所示,1mol四异丙醇钛中含有σ键的数目为___mol。
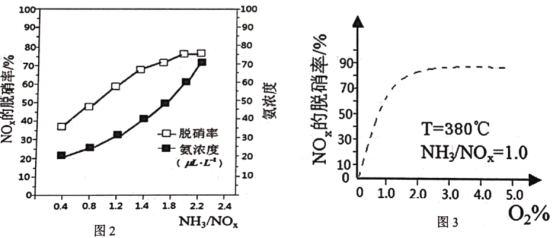
(4)偏钛酸钡的晶体结构如图-2所示,则偏钛酸钡的化学式为___;与Ba2+最近且等距离的O2-为___个。
【答案】[Ar]3d24s2或1s22s22p63s23p63d24s2 平面正三角形 SO3或BF3 44 BaTiO3 12
【解析】
(1)Ti是22号元素,位于第四周期,其基态核外电子排布式为:1s22s22p63s23p63d24s2或[Ar]3d24s2,故答案为:[Ar]3d24s2或1s22s22p63s23p63d24s2;
(2)碳酸根离子中价层电子对个数=![]() =3,且没有孤电子对,所以是平面正三角形,原子个数和价电子数与CO32-相等的分子是CO32-的等电子体,所以可以用S换了C,得SO3,也可以用F换了O,用B换了C,得BF3,故答案为:平面三角形;SO3或BF3;
=3,且没有孤电子对,所以是平面正三角形,原子个数和价电子数与CO32-相等的分子是CO32-的等电子体,所以可以用S换了C,得SO3,也可以用F换了O,用B换了C,得BF3,故答案为:平面三角形;SO3或BF3;
(3)单键全是σ键,1个C12H28O4Ti分子中含44个单键,故1mol四异丙醇钛(C12H28O4Ti)中含有44molσ键,故答案为:44;
(4)均摊法可知:Ba2+个数=8×![]() =1,O2-个数=6×
=1,O2-个数=6×![]() =3,Ti4+个数=1,所以偏钛酸钡的化学式为:BaTiO3,与与Ba2+最近且等距离的O2-为晶胞中立方体的面对角线的交点处的O2-,从图上可看出,1个晶胞中这样的O2-有3个,按照均摊法计算,实际有=3
=3,Ti4+个数=1,所以偏钛酸钡的化学式为:BaTiO3,与与Ba2+最近且等距离的O2-为晶胞中立方体的面对角线的交点处的O2-,从图上可看出,1个晶胞中这样的O2-有3个,按照均摊法计算,实际有=3![]() =1.5个,所以总的O2-有1.5×8=12个,故答案为:BaTiO3;12。
=1.5个,所以总的O2-有1.5×8=12个,故答案为:BaTiO3;12。

 红果子三级测试卷系列答案
红果子三级测试卷系列答案 课堂练加测系列答案
课堂练加测系列答案【题目】下表是不同温度下水的离子积的数据:
温度 | 25 |
|
|
水的离子积 |
| a |
|
试回答以下问题
(1)若![]() ,则a______1×10-14填“<”“>”或“=”)。
,则a______1×10-14填“<”“>”或“=”)。
(2)250C时,某Na2SO4溶液中c(SO42-)=5×10-4mol/L,取该溶液1mL加水稀释至10mL,则稀释后溶液中![]() :
:![]() ______。
______。
(3)在![]() 温度下测得某溶液
温度下测得某溶液![]() ,该溶液显______
,该溶液显______![]() 填“酸”、“碱”或“中”
填“酸”、“碱”或“中”![]() 性
性![]() 将此温度下
将此温度下![]() 的NaOH溶液aL与
的NaOH溶液aL与![]() 的
的![]() 溶液bL混合,若所得混合液
溶液bL混合,若所得混合液![]() ,则a:b______。
,则a:b______。
(4)某同学用0.1 mol·L-1的NaOH溶液分别滴定20.00 mL0.1 mol·L-1的HCl溶液和0.1 mol·L-1的CH3COOH溶液,得到如图所示的两条滴定曲线,请回答有关问题:
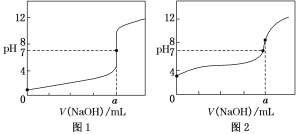
①0.1 mol·L-1的NaOH溶液滴定0.1 mol·L-1的CH3COOH溶液的曲线是______(填“图1”或“图2”)曲线。
②a=________。
(5)某同学用0.1 mol·L-1的NaOH溶液分别滴定20.00 mL未知浓度的HCl溶液选用_______作指示剂,若装标准液的滴定管未润洗则会导致测定结果________(填“偏高”或“偏低”或“无影响”)
【题目】苯甲酸乙酯(C9H10O2)的别名为安息香酸乙酯。它是一种无色透明液体,不溶于水,有芳香气味,用于配制香水、香精和人造精油,大量用于食品工业中,也可用作有机合成中间体、溶剂等。其制备方法为: ![]() +CH3CH2OH
+CH3CH2OH![]()
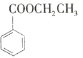 +H2O
+H2O
已知:苯甲酸在100℃会迅速升华。相关有机物的性质如表所示。
名称 | 相对分子质量 | 颜色、状态 | 沸点/℃ | 密度/(g﹒cm-3) |
苯甲酸 | 122 | 无色片状晶体 | 249 | 1.2659 |
苯甲酸乙酯 | 150 | 无色澄清液体 | 212.6 | 1.05 |
乙醇 | 46 | 无色澄清液体 | 78.3 | 0.7893 |
环己烷 | 84 | 无色澄清液体 | 80.8 | 0.7318 |
实验步骤如下:
①在圆底瓶中加入12.20g苯甲酸,25mL95%的乙醇(过量),20mL环己烷以及4mL浓硫酸,混合均匀并加入沸石,按如图所示装置装好仪器,控制温度在65~70℃加热回流2h。利用分水器不断分离除去反应生成的水,回流环己烷和乙醇。

②反应结束,打开旋塞放出分水器中的液体后,关闭旋塞,继续加热,至分水器中收集到的液体不再明显增加,停止加热。
③将烧瓶内反应液倒入盛有适量水的烧杯中,分批加入Na2CO3至溶液呈中性。用分液漏斗分出有机层,水层用25mL乙醚,静置,过滤,对滤液进行蒸馏,低温蒸出乙醚和环己烷后,继续升温,接收210~213℃的馏分。
④检验合格,测得产品体积为12.86mL。
回答下列问题:
(1)在该实验中,圆底烧瓶的容积最适合的是________(填序号)。
A. 25mL B.50mL C. 100mL D. 250mL
(2)步骤①中使用分水器不断分离除去水的目的是___________。
(3)步骤②中应控制蒸馏的温度为_______(填序号)。
A. 65~70℃ B. 78~80℃ C. 80~85℃ D. 215~220℃
(4)步骤③加入Na2CO3的作用是_______;若Na2CO3的加入量不足,在之后蒸馏时,蒸馏烧瓶中可见到白烟生成,产生该现象的原因是________。
(5)关于步骤③中的萃取分液操作的叙述正确的是__________(填序号)。
A. 水溶液中加入乙醚,转移至分液漏斗中,塞上玻璃塞,分液漏斗倒转过来,用力振摇
B. 振摇几次后需打开分液漏斗上口的玻璃塞放气
C. 经几次振摇并放气后,手持分液漏斗静置待液体分层
D. 放出液体时,应打开上口玻璃塞或将玻璃塞上的凹槽对准漏斗口上的小孔
(6)计算可得本实验的产率为__________。