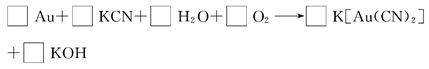��Ŀ����
����ͼ��ʯī���缫�ĵ����У�����500mL��һ�����ʵ�ij��ɫ��������Һ���е�⣬�۲쵽A�缫�����к�ɫ�Ĺ�̬�������ɣ�B�缫����ɫ�������ɡ���ش��������⣺
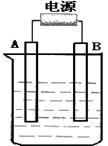
��1����д��B��������ƣ� �缫��Ӧʽ
д�����ʱ��Ӧ�������ӷ���ʽ
��2��������Һ�е�ԭ��������ȫ����ֹͣ��⣬ȡ��A�缫��ϴ�ӡ�����������缫����1.6g��������Һ��pHΪ ��Ҫʹ������Һ�ָ������ǰ��״̬��������� ��������Ϊ g����������ǰ����Һ��������䣩
��3����ԭ��ҺΪ1L K2SO4��CuSO4�Ļ����Һ����c��SO42-��=" 2.0mol/L" ����ͼװ�õ�⣬���������ռ���22.4L���壨��״����ʱ��ֹͣ��⡣
��ԭ��Һ�е�c(K+)��

��1����д��B��������ƣ� �缫��Ӧʽ
д�����ʱ��Ӧ�������ӷ���ʽ
��2��������Һ�е�ԭ��������ȫ����ֹͣ��⣬ȡ��A�缫��ϴ�ӡ�����������缫����1.6g��������Һ��pHΪ ��Ҫʹ������Һ�ָ������ǰ��״̬��������� ��������Ϊ g����������ǰ����Һ��������䣩
��3����ԭ��ҺΪ1L K2SO4��CuSO4�Ļ����Һ����c��SO42-��=" 2.0mol/L" ����ͼװ�õ�⣬���������ռ���22.4L���壨��״����ʱ��ֹͣ��⡣
��ԭ��Һ�е�c(K+)��
��1��������2�֣�4OH-- 4e-=2H2O+O2 ����2�֣���
2Cu2+ +2H2O =" " 2Cu +O2 ��+ 4H+��2�֣�
��2��PH=1��2�֣� CuO ��2�֣�; 2�ˣ�2�֣� ����CuCO3 3.1 ��
��3�� c��K+��="2mol/L " ��3�֣�
|
2Cu2+ +2H2O =" " 2Cu +O2 ��+ 4H+��2�֣�
��2��PH=1��2�֣� CuO ��2�֣�; 2�ˣ�2�֣� ����CuCO3 3.1 ��
��3�� c��K+��="2mol/L " ��3�֣�
��

��ϰ��ϵ�д�
�����Ŀ
 ��Һʱ��ͼ12-5��ʾ�ĵ��仯���ߺ�������
��Һʱ��ͼ12-5��ʾ�ĵ��仯���ߺ�������
 ��״��������֪��Au��HNO3��4HCl===H[AuCl4]��NO����2H2O��
��״��������֪��Au��HNO3��4HCl===H[AuCl4]��NO����2H2O��