��Ŀ����
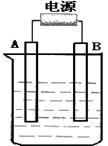
��8�֣�(1998���Ϻ�)�ȼ��ⱥ��ʳ��ˮ��ȡNaOH�Ĺ�������ʾ��ͼ����
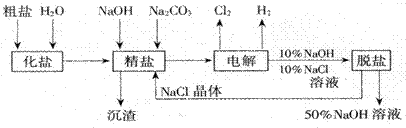
������ͼ�����������գ�
(1)�ڵ������У����Դ���������ĵ缫����������Ӧ�Ļ�ѧ����ʽΪ �����Դ���������ĵ缫��������ҺpH (ѡ����䡱�����ߡ����½���)��
(2)��ҵʳ�κ�Ca2+��Mg2+�����ʡ����ƹ��̷�����Ӧ�����ӷ���ʽΪ
�� ��
(3)���������SO42-�����ϸߣ��������ӱ��Լ���ȥSO42-���ñ��Լ�����
�� (ѡ��a��b��c��ѡ�۷�)��
a��Ba(OH)2 b��Ba(NO3)2 c��BaCl2
(4)Ϊ��Ч��ȥCa2+��Mg2+��SO42���������Լ��ĺ���˳��Ϊ (ѡ��a��b��c��ѡ�۷�)
a���ȼ�NaOH,���Na2CO3���ټӱ��Լ�
b���ȼ�NaOH����ӱ��Լ����ټ�Na2CO3
c���ȼӱ��Լ������NaOH���ټ�Na2CO3
(5)���ι���������NaOH��NaCl���ܽ���ϵIJ��죬ͨ�� ����ȴ�� (��д��������)��ȥNaCl
(6)�ڸ�Ĥ�����ʳ��ˮʱ�����۷ָ�Ϊ������������������ֹCl2��NaOH��Ӧ��������Ĥ������ʳ��ˮʱ��Cl2��NaOH��ֽӴ����������NaClO��H2����Ӧ�Ļ�ѧ����ʽΪ ��

������ͼ�����������գ�
(1)�ڵ������У����Դ���������ĵ缫����������Ӧ�Ļ�ѧ����ʽΪ �����Դ���������ĵ缫��������ҺpH (ѡ����䡱�����ߡ����½���)��
(2)��ҵʳ�κ�Ca2+��Mg2+�����ʡ����ƹ��̷�����Ӧ�����ӷ���ʽΪ
�� ��
(3)���������SO42-�����ϸߣ��������ӱ��Լ���ȥSO42-���ñ��Լ�����
�� (ѡ��a��b��c��ѡ�۷�)��
a��Ba(OH)2 b��Ba(NO3)2 c��BaCl2
(4)Ϊ��Ч��ȥCa2+��Mg2+��SO42���������Լ��ĺ���˳��Ϊ (ѡ��a��b��c��ѡ�۷�)
a���ȼ�NaOH,���Na2CO3���ټӱ��Լ�
b���ȼ�NaOH����ӱ��Լ����ټ�Na2CO3
c���ȼӱ��Լ������NaOH���ټ�Na2CO3
(5)���ι���������NaOH��NaCl���ܽ���ϵIJ��죬ͨ�� ����ȴ�� (��д��������)��ȥNaCl
(6)�ڸ�Ĥ�����ʳ��ˮʱ�����۷ָ�Ϊ������������������ֹCl2��NaOH��Ӧ��������Ĥ������ʳ��ˮʱ��Cl2��NaOH��ֽӴ����������NaClO��H2����Ӧ�Ļ�ѧ����ʽΪ ��
(1)2Cl-��2e��====Cl2������(2)Ca2++CO32��=====CaCO3��Mg2++2OH-====Mg(OH)2��
(3)a��c (4)b��c (5)���������� (6)NaCl+H2O=====NaClO+H2����2NaCl+2H2O=======H2��+Cl2��+2NaOH Cl2+2NaOH====NaCl+NaClO+H2O
(3)a��c (4)b��c (5)���������� (6)NaCl+H2O=====NaClO+H2����2NaCl+2H2O=======H2��+Cl2��+2NaOH Cl2+2NaOH====NaCl+NaClO+H2O
��

��ϰ��ϵ�д�
�����Ŀ