��Ŀ����
16��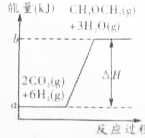 ����ҵ���ƶ���������һ���¶ȣ�230��280�棩��ѹǿ��2.0��10.0MPa���ʹ������ý��еģ���Ӧ���з��������з�Ӧ��
����ҵ���ƶ���������һ���¶ȣ�230��280�棩��ѹǿ��2.0��10.0MPa���ʹ������ý��еģ���Ӧ���з��������з�Ӧ��CO��g��+2H2��g��?CH3OH��g������H1=-90.7kJ•mol-1��
2CH3OH��g��?CH3OCH3��g��+H2O��g������H2=-23.5kJ•mol-1��
CO��g��+H2O?��g��CO2��g��+H2��g������H3=-41.2kJ•mol-1��
��1����Ӧ���е��ܷ�Ӧ�ɱ�ʾΪ3CO��g��+3H2��g��?CH3OCH3��g��+CO2��g�����÷�Ӧ�ġ�H=-246.1kJ/mol�����ܱ������У���ʼͶ��3molCO��3molH2����һ�������´ﵽƽ�⣮�÷�Ӧ�ų�������ΪС��246.1kJ���������ɣ���Ϊ��Ӧ�ǿ���ģ����ܽ�����ȫ��
��2��������̼ʹ�����к�����ߵ�һ���������壬���ƺ�����������̼�ǽ������ЧӦ����Ч;����Ŀǰ���ɶ�����̼�ϳɶ�������ȡ���˽ϴ�Ľ�չ����ѧ��Ӧ��������ϵ��ͼ��ʾ��д���÷�Ӧ���Ȼ�ѧ����ʽ��CO2��g��+6H2��g���TCH3OCH3��g��+3H2O��g����H=��b-a��kJ/mol��
���� ��1���ɷ�Ӧ��CO��g��+2H2��g��?CH3OH��g����H=-90.7kJ•mol-1����2CH3OH��g��?CH3OCH3��g��+H2O��g����H=-23.5kJ•mol-1����CO��g��+H2O��g��?CO2��g��+H2��g����H=-41.2kJ•mol-1�����١�2+��+�۵�3CO��g��+3H2��g��?CH3OCH3��g��+CO2��g�����ݸ�˹���ɿɼ��㷴Ӧ�ġ�H��
��2�����ݸ�˹���ɽ���Ȼ�ѧ����ʽ����д֪ʶ�����лش�
��� �⣺��1������֪����CO��g��+2H2��g��?CH3OH��g����H=-90.7kJ/mol����2CH3OH��g��?CH3OCH3��g��+H2O��g����H=-23.5kJ/mol����CO��g��+H2O��g��?CO2��g��+H2��g����H=-41.2kJ/mol���ɸ�˹���ɿ�֪���١�2+��+�۵�3CO��g��+3H2��g��?CH3OCH3��g��+CO2��g����H=-246.1kJ/mol�����ܱ������У���ʼͶ��3molCO��3molH2����һ�������´ﵽƽ�⣬�÷�Ӧ�ų�������С��246.1kJ����Ϊ��Ӧ�ǿ���ģ����ܽ�����ȫ��
�ʴ�Ϊ��-246.1kJ/mol��С��246.1kJ����Ϊ��Ӧ�ǿ���ģ����ܽ�����ȫ��
��2������ͼʾ��Ϣ�õ��ɶ�����̼�ϳɶ����ѵķ�Ӧ�����ȵģ��÷�Ӧ���Ȼ�ѧ����ʽΪ��CO2��g��+6H2��g���TCH3OCH3��g��+3H2O��g����H=��b-a��kJ/mol���ʴ�Ϊ��CO2��g��+6H2��g���TCH3OCH3��g��+3H2O��g����H=��b-a��kJ/mol��
���� ���⿼��ѧ����˹���ɵ�Ӧ���Լ��Ȼ�ѧ����ʽ����д֪ʶ��ע��֪ʶ�Ĺ��ɺ������ǽ���Ĺؼ����Ѷ��еȣ�

 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�| A�� | HCO3-+H2O?H3O++CO32- | B�� | NH3+H2O?NH4++OH- | ||
| C�� | CO32-+2H2O?H2CO3+2OH- | D�� | Al3++3H2O?Al��OH��3+3H+ |
| A�� | �Եع���Ϊԭ�ϼӹ��Ƴɵ�������͵ijɷ����ʯ�ͷ���õ��IJ��ͳɷֲ�ͬ | |
| B�� | ��ʳƷ���з���ʢ�й轺�����۵���С�����ɷ�ֹʳ���ܳ����������� | |
| C�� | ��ɫ��ѧ�ĺ�����Ӧ�û�ѧԭ���Ի�����Ⱦ�������� | |
| D�� | ��Ȼ����������ˮú���ֱ����ڻ�ʯ��Դ����������Դ�Ͷ�����Դ |
 ���������dz��õ�ҽҩ�м��壬���ɱ����������Ʊ�����Ӧ�Ļ�ѧ����ʽ���£�
���������dz��õ�ҽҩ�м��壬���ɱ����������Ʊ�����Ӧ�Ļ�ѧ����ʽ���£�
�йػ�������������ʼ��±���
| ������ | �ܶȣ�g��cm-3�� | �ܽ��� | �۵㣨�棩 | �е㣨�棩 |
| ���� | 1.05 | ������ˮ���Ҵ� | 17 | 118 |
| ���� | 1.02 | ����ˮ���������Ҵ� | -6 | 184 |
| �������� | - | ������ˮ����������ˮ���������Ҵ� | 114 | 304 |
�����ף�����װ�üף�������������������ʯ�ͷ����еķ�����������Բ����ƿ�м���5��0mL ������7.4mL���ᣬ�������У������¶ȼƶ���100��105�棬����Һ��ƽ����������Ӧ40min��ֹͣ���ȣ���Բ����ƿ�е�Һ����ȵ���ʢ��100mLˮ���ձ�����ȴ�������������������������˵ôֲ��
�����ң�����װ���ң����Ȼ�������Ӧ40min��ֹͣ���ȣ������뷽������ͬ��
�ᴿ�������������������ؽᾧ�������������£�
�ټ����ܽ���ڻ���̿��ɫ���۳��ȹ��ˡ�����ȴ�ᾧ���ݹ��ˡ���ϴ�ӡ��߸���
��ش�
��1������a�������������ܣ�b��ˮ�������dz�ˮ�����ˮ����ˮ������
��2���ϳɲ����У��������������������˷�����ֲ��������Һ�е���Ҫ���������ᣮ
��3���ᴿ�����еĵڢ۲�������Ҫ���ȵ������Ƿ�ֹ�¶Ƚ��͵��������������������Ͳ��ʣ�
��4���ᴿ���̵ڢ�ϴ�ӣ�����ϴ�Ӽ�������ʵ���A��
A������ˮ B���Ҵ� C��5%Na2CO3��Һ D������NaCl��Һ
��5����Ͷ����������Ϊ��������������ʣ��������ַ�������ȡ�Ĵ�ʩ�����������ʵ�������������IJ��ʽϸߣ�ԭ���Ƿ�������Ӧ���������ɵ�ˮ�������ٽ���Ӧ��

| A�� | Zn2++2e-�TZn | B�� | Cu2++2e-�TCu | C�� | Zn-2e-�TZn2+ | D�� | 2H++2e-�TH2�� |
| A�� | ̼�ᱵ����������θ������ҩ | |
| B�� | �������������ʳƷ��Ư�� | |
| C�� | ̼��ά�Ǹ��ϲ��� | |
| D�� | PM2.5ָ����������������е���Ҫָ�� |
| ѡ�� | ʵ��Ŀ�� | ʵ����� |
| A | ֤������ԭ����ܼӿ���ȡ�������� | ��п��ϡ���ᷴӦʱ����������ͭ |
| B | ֤����������������Ӧ������������ԭ��Ӧ | ��пƬ��ͭƬ�ֱ��������ͭ��Һ�� |
| C | ֤���缫������������Һ�й� | ������þΪ�缫���ֱ�������������Һ��ϡ�����й���ԭ��� |
| D | ֤��Ag+�������Ա�Cu2+ǿ | ��ʯīΪ�缫�����0.001mol•L-1AgNO3��Һ��1mol•L-Cu��NO3��2��Һ |
| A�� | A | B�� | B | C�� | C | D�� | D |
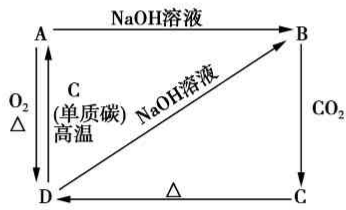 ��֪A�ǻҺ�ɫ���н�������Ĺ��嵥�ʣ�������ͼ��ʾ������֮���ת����ϵ���ش������й����⣮ ��1��д��B��C�����ʵ����ƣ�B�����ƣ�C���ᣮ
��֪A�ǻҺ�ɫ���н�������Ĺ��嵥�ʣ�������ͼ��ʾ������֮���ת����ϵ���ش������й����⣮ ��1��д��B��C�����ʵ����ƣ�B�����ƣ�C���ᣮ