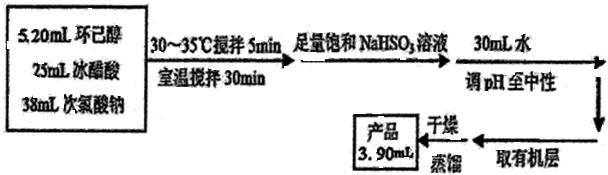
��Ŀ����
17��ijǿ������ҺX�н�����Ba2+��Al3+��NH4+��Fe2+��Fe3+��CO32-��SO32-��SO42-��Cl-��NO3-�е�һ�ֻ��֣�ȡ����Һ��������ʵ�飬ʵ��������£�
����������Ϣ���ش��������⣺
��1����������������ʵ�鲻��ȷ����ҺX���Ƿ��е����������ӷֱ���Fe3+��Cl-����Ҫ��ʵ��֤�����������Ƿ���ڣ���ɿ��Ļ�ѧ������ȡ����B��Һ���Թ��У������еμ�AgNO3��Һ�����а�ɫ�������ɣ�˵������Cl-������Cl-�����ڣ�
��2�����������ӷ��̱������з�Ӧ��
������������A��3Fe2++4H++NO3-=3Fe3++NO��+2H2O��
����������ҺH��Al3++4OH-=AlO2-+2H2O��
���в���������F�������������̣�8NH3+3Cl2=6NH4Cl+N2��
��3��������������������Ũ��Ϊ2mol/L��������l0mlʱ��ʼ����������55mlʱ���������ﵽ���ֵ0.03mol�������μӳ����������ֲ��䣬����ٵμӳ��������ܽ⣬��60mlʱ����������Ϊ0.025mol�ұ��ֲ��䣬��ԭ��Һ��c��Fe2+��Ϊ0.15mol/L��c��Fe3+��Ϊ0.1mol/L��c��H+��Ϊ0.4mol/L��c��Cl-��Ϊ0.4mol/L��������Щ���Ӳ����ڣ�����0mol/L��
���� ��ǿ������Һ��һ���������CO32-��SO32-���ӣ�����������ᱵ���ɳ�������ó���CΪBaSO4��˵����Һ�к���SO42-���ӣ��������ᱵ���������Լ�����������ӵ����ʵ�������������A��A������������D��E����AΪNO��DΪNO2��EΪHNO3��˵����Һ�к��л�ԭ�����ӣ���һ��ΪFe2+���ӣ�����NO��������Լ����������ӵ�������ҺB�м������NaOH��Һ������GֻΪFe��OH��3����������F����FΪNH3��˵����Һ�к���NH4+���ӣ���ҺH��ͨ��CO2���壬���ɳ���I����IΪAl��OH��3��HΪNaOH��NaAlO2��˵����Һ�к���Al3+���ӣ��ٸ������ӹ���֪ʶ����Һ�к���Fe2+���ӣ���һ������NO3-���ӣ�����SO42-���Ӿ�һ������Ba2+���ӣ�����ȷ���Ƿ��е�����Fe3+��Cl-���Դ˽��н��
��� �⣺ǿ������Һ��һ���������CO32-��SO32-���ӣ�����������ᱵ���ɳ�������ó���C9.32gΪBaSO4��˵����Һ�к���SO42-���ӣ��������ᱵ���������Լ�����������ӵ����ʵ���Ϊ$\frac{9.32g}{233g/mol}$=0.04mol��Ũ����$\frac{0.04mol}{0.1L}$=0.4mol/L����������A��A������������D��E����AΪNO��DΪNO2��EΪHNO3��˵����Һ�к��л�ԭ�����ӣ���һ��ΪFe2+���ӣ�����NO��������Լ����������ӵ�������ҺB�м������NaOH��Һ������GֻΪFe��OH��3����������F����FΪNH3��112mL���������ʵ�����0.005mol�����ݵ�Ԫ���غ㣬��Һ�к���NH4+�������ʵ�����0.005mol��Ũ����0.05mol/L����ҺH��ͨ��CO2���壬���ɳ���I����IΪAl��OH��3��HΪNaOH��NaAlO2��˵����Һ�к���Al3+���ӣ��ٸ������ӹ���֪ʶ����Һ�к���Fe2+���ӣ���һ������NO3-���ӣ�����SO42-���Ӿ�һ������Ba2+���ӣ�����ȷ���Ƿ��е�����Fe3+��Cl-��
��1���������Ϸ�����֪����Һ�в���ȷ����������ΪFe3+����Һ�в���ȷ����������ΪCl-�����������ӵķ���Ϊ��ȡ����B��Һ���Թ��У������еμ�AgNO3��Һ�����а�ɫ�������ɣ�˵������Cl-������Cl-�����ڣ�
�ʴ�Ϊ��Fe3+��Cl-��ȡ����B��Һ���Թ��У������еμ�AgNO3��Һ�����а�ɫ�������ɣ�˵������Cl-������Cl-�����ڣ�
��2�����еõ�������A��NO��Fe2+���ӱ�����ΪFe3+���ӣ�NO3-���ӱ���ԭΪNO���壬��Ӧ�����ӷ���ʽΪ��3Fe2++NO3-+4H+=3Fe3++NO��+2H2O��
�ʴ�Ϊ��3Fe2++4H++NO3-=3Fe3++NO��+2H2O��
��H��ƫ��������Һ��������������������Ƶķ�Ӧ������ƫ��������Ӻ�ˮ�����ӷ���ʽ��Al3++4OH-=AlO2-+2H2O��
�ʴ�Ϊ��Al3++4OH-=AlO2-+2H2O��
��FΪNH3������������������Ӧ�����Ȼ�狀͵�������Ӧ�Ļ�ѧ����ʽΪ��8NH3+3Cl2=6 NH4Cl+N2��
�ʴ�Ϊ��8NH3+3Cl2=6 NH4Cl+N2��
��3�����ݷ�Ӧ��3Fe2++NO3-+4H+=3Fe3++NO��+2H2O���õ�112mL0.005molNO��Fe2+�����ʵ�����0.015mol������ԭ��Һ��c��Fe2+��=$\frac{0.015mol}{0.1L}$=0.15mol/L����������������60mlʱ������������Ϊ0.025mol�����������������ʵ�����0.025mol��������Ԫ���غ㣬����Fe3+�����ʵ�����0.01mol������ԭ��Һ��c��Fe3+��=$\frac{0.01mol}{0.1L}$=0.1mol/L����������ӵ����ʵ���Ϊ$\frac{9.32g}{233g/mol}$=0.04mol��Ũ����$\frac{0.04mol}{0.1L}$=0.4mol/L��NH4+�������ʵ�����0.005mol��Ũ����0.05mol/L��Al3+�����ʵ�����0.005mol��Ũ����0.05mol/L��
���ݷ�Ӧ��3Fe2++NO3-+4H+=3Fe3++NO��+2H2O����֪�μӷ�ӦH+Ϊ0.005mol��4=0.02mol������������������H+Ϊ0.01L��2mol/L=0.02mol����ԭ��Һ��H+Ϊ0.02mol+0.02mol=0.04mol������2c��Fe2+��+3c��Fe3+��+3c��Al3+��+c��NH4+��=��2��0.15+3��0.1+3��0.05+0.05��mol/L=0.8mol/L��2c��SO42-��=0.8mol/L��ԭ��Һ�к���Cl-�����ݵ���غ㣺c��Cl-��=c��H+��=$\frac{0.04mol}{0.1L}$=0.4mol/L��
�ʴ�Ϊ��0.15�� 0.1��0.4��0.4��
���� ���⿼���˳����������ӵļ��顢���ƶϣ���Ŀ�Ѷ��еȣ�ע�����ճ������ӵ����ʼ����鷽������3��Ϊ�ѵ㡢�״��㣬ע����Һ������֪ʶ�ڻ�ѧ�����е�Ӧ�÷����������ֿ�����ѧ���ķ������������������Ӧ����ѧ֪ʶ��������

��1��ijͬѧ���ø÷�Ӧ̽��Ũ�ȶԷ�Ӧ���ʵ�Ӱ�죮ʵ��ʱ������1mL������Һ��ָʾ�������������㣬ֱ��ͨ����ɫʱ��ij����ж�Ũ���뷴Ӧ���ʵĹ�ϵ�������Լ���Ӧѡ��ڢۢܣ�����ţ���
��1mL 0.01mol•L-1�ĵ�ˮ ��1mL 0.001mol•L-1�ĵ�ˮ ��4mL 0.01mol•L-1��Na2S2O3��Һ ��4mL 0.001mol•L-1��Na2S2O3��Һ
��2����ijͬѧѡȡ�٢۽���ʵ�飬�����ɫʱ��Ϊ4s������v��S2O${\;}_{3}^{2-}$��=8.33��10-4mol/��L•s����
��3���������������ú��ˮ��ԭ�Ͼ��ಽ��Ӧ�Ƶã����е�һ����ӦΪ��CO��g��+H2O��g��?CO2��g��+H2��g����H��0��200��ʱ���÷�Ӧ��ƽ�ⳣ��Ϊ$\frac{1}{2.25}$�����¶��£���һ������CO��H2Ͷ��ij10L�ܱ�������5minʱ��ƽ�⣬������Ũ�ȣ�mol
•L-1���仯�����
| 0min | 5min | 10min | |
| CO | 0.01 | 0.0056 | |
| H2O | 0.01 | 0.0156 | |
| CO2 | 0 | 0.0044 | |
| H2 | 0 | 0.0044 |
��CO��ƽ��ת����Ϊ40%��
����5min��10minֻ�ı���ijһ������������������ˮ������Ũ�ȣ���θı������0.01mol/L��
| A�� | ��һ������YС��X | |
| B�� | ��ۺ���������ԣ�X��Ӧ���������ǿ��Y | |
| C�� | ��̬�⻯����ȶ��ԣ�HmYǿ��HnX | |
| D�� | X��Y�γɻ�����ʱ��X�Ը��ۣ�Y������ |
| A�� | ��Һ����ȡ������ | B�� | ��ȡ������Һ | C�� | ������ȡ����Һ | D�� | ��Һ��������ȡ |
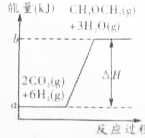 ����ҵ���ƶ���������һ���¶ȣ�230��280�棩��ѹǿ��2.0��10.0MPa���ʹ������ý��еģ���Ӧ���з��������з�Ӧ��
����ҵ���ƶ���������һ���¶ȣ�230��280�棩��ѹǿ��2.0��10.0MPa���ʹ������ý��еģ���Ӧ���з��������з�Ӧ�� ���ǵؿ��к������Ľ���Ԫ�أ��䵥�ʺͻ�����㷺Ӧ�����ճ������У�
���ǵؿ��к������Ľ���Ԫ�أ��䵥�ʺͻ�����㷺Ӧ�����ճ������У� ����ͪ����Ҫ����ԭ�ϣ����������������������ͼ��������Ҫ�м��壮ij��ѧ��ȤС�鳢���ô��������Ʊ�����ͪ������ʽΪ��
����ͪ����Ҫ����ԭ�ϣ����������������������ͼ��������Ҫ�м��壮ij��ѧ��ȤС�鳢���ô��������Ʊ�����ͪ������ʽΪ��