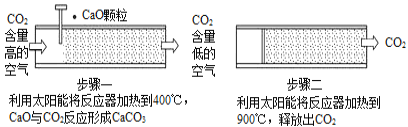
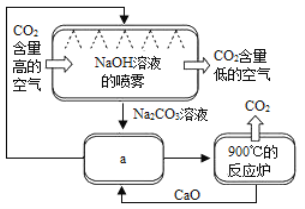
��Ŀ����
����Ŀ���������������ʵ���Һ����Na2CO3 ��Al2(SO4)3 ��CH3COOH ��NaHCO3��
��1��д������Һ�ĵ���غ㣺____________��
��2�������£�0.1 mol��L-1����Һ��pH����8������Һ��c(H2CO3)_____c(CO32��)�����������=����������ԭ����_______�������ӷ���ʽ�ͱ�Ҫ������˵������
��3��������0.1 mol/L�Ģ���Һ��ˮϡ�����У����б���ʽ������һ��������_________��
A��c(H��) B��c(H+)/c(CH3COOH) C��c(H��)��c(OH��)
��4���âںܵ͢���Һ����������ĭ��������ԭ��Ϊ��________________�������ӷ���ʽ���ͣ�
���𰸡�C(Na+)+C(H+)=2C(CO32-)+C(HCO3-)+C(OH-) > HCO3- +H2O![]() H2CO3+OH-��HCO3-
H2CO3+OH-��HCO3-![]() H+ +CO32- ���� HCO3- ��ˮ����� HCO3- �ĵ��� B Al3+ +3HCO3- =Al(OH)3�� +3 CO2��
H+ +CO32- ���� HCO3- ��ˮ����� HCO3- �ĵ��� B Al3+ +3HCO3- =Al(OH)3�� +3 CO2��
��������
��Na2CO3��Һ�ĵ���غ㣬����������������ɵ���������������ɣ�c(Na+)+c(H+)= 2c(CO32��)+c(HCO3��)+c(OH��)���ʴ�Ϊc(Na+)+c(H+)=2c(CO32��)+c(HCO3��)+c(OH��)��
�Ƴ����£�0.1 mol��L-1 NaHCO3��Һ��pH����8����ˮ�ⷽ��ʽΪ
HCO3��+H2O![]() H2CO3+OH����HCO3��
H2CO3+OH����HCO3��![]() H+ +CO32����HCO3����Ҫ���룬��Ҫˮ�⣬pH����8��˵��ˮ��Ϊ����HCO3��+H2O
H+ +CO32����HCO3����Ҫ���룬��Ҫˮ�⣬pH����8��˵��ˮ��Ϊ����HCO3��+H2O![]() H2CO3+OH��(��)��HCO3��
H2CO3+OH��(��)��HCO3��![]() H+ +CO32��(��)��������Һ��c(H2CO3)��c(CO32��)���ʴ�Ϊ����HCO3��+H2O
H+ +CO32��(��)��������Һ��c(H2CO3)��c(CO32��)���ʴ�Ϊ����HCO3��+H2O![]() H2CO3+OH��(��)��HCO3��
H2CO3+OH��(��)��HCO3��![]() H+ +CO32��(��)������ HCO3����ˮ����� HCO3���ĵ���
H+ +CO32��(��)������ HCO3����ˮ����� HCO3���ĵ���
�dz�����0.1 mol/L��CH3COOH��Һ��ˮϡ�����У�ƽ�������ƶ�����������Һ�������ռ��Ҫ�����c(H��)��С��c(H��)��c(OH��) = KW���䣬��ΪKWֻ���¶��йأ�![]() ��KW���䣬c(CH3COO��)��С������
��KW���䣬c(CH3COO��)��С������![]() ���ʴ�ΪB��
���ʴ�ΪB��
����Al2(SO4)3��NaHCO3����Һ����������ĭ��������Ҫԭ�������߷���˫ˮ�������������������Ͷ�����̼���壬��ԭ��ΪAl3+ +3HCO3�� =Al(OH)3�� +3 CO2�����ʴ�ΪAl3+ +3HCO3�� =Al(OH)3�� +3 CO2����

 ͬ��������ϰϵ�д�
ͬ��������ϰϵ�д� �ο�ͨ�γ̱�˼ά����������ѵ��ϵ�д�
�ο�ͨ�γ̱�˼ά����������ѵ��ϵ�д�����Ŀ��������Ϣд�����з�Ӧ�ķ���ʽ�������ʵ����ʡ�
����������[In(OH)3]�㷺Ӧ���ڵ�����ҵ������������������Ҫ��In2O3��Fe3O4��Ϊԭ���Ʊ�����������һ�ֹ����������£�
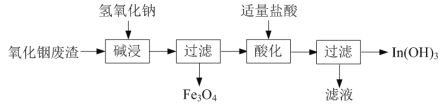
��֪��In2O3Ϊ�����������ǿ����������InԪ����InO33-������ʽ���ڡ�
��д���������ʱ��Ӧ�����ӷ���ʽ��______��
��д�����ữ��ʱ��Ӧ�����ӷ���ʽ��______��
���±���ʾΪ������ĵ���ƽ�ⳣ����
�� | H2SO3 | CH3COOH | HCOOH |
����ƽ�ⳣ�� | Ka1��1.2��10��2 Ka2��5.6��10��8 | 1.75��10��5 | 1.8��10��4 |
��H2SO3��CH3COOH��ѡ����ʵ����ʣ���ʵ��HCOONa��HCOOH��ת����д���÷�Ӧ�����ӷ���ʽ��______��
���±���ʾΪ�������ʵ��ܶȻ�������
������ | Mn(OH)2 | Co(OH)2 | MnCO3 |
�ܶȻ����� | 2��10��13 | 5.9��10��15 | 2.2��10��11 |
�ٴ�NaOH��Co(OH)2��ѡȡ���ʵ����ʣ���������ת����ϵ�У�MnCl2��Mn(OH)2��______
��NaNO3��Na2CO3��ѡ����ʵ����ʣ���������ת����ϵ�У�MnCl2��NaCl��_______
����Ŀ�����и���ʵ������������ͻ���۾���ȷ���ǣ� ����
���� | ���� | ���ͻ���� | |
A | ��ʯ��������ͨ�����ȵ����Ƭ����������ͨ��������Ȼ�̼��Һ | ������Ȼ�̼��Һ����ɫ | �����ֽ������ϩ�����巢����������Ӧ |
B | ������״ͭ˿�ھƾ��������������Ȳ����Ҵ��� | ͭ˿�ָ��������Ϻ�ɫ�ҿ��ŵ��̼�����ζ | ͭ˿���������Ҵ�����������ȩ |
C | ���Ҵ������ᡢŨ�����Ϲ��Ȳ���������ͨ������ | Һ���������IJ�����ˮ����״Һ�����ɣ��������ŵ���ζ | Ũ���������������������£��Ҵ������ᷢ����������Ӧ |
D | ������Һ������ϡ�����ˮԡ���ȼ����ӣ�Ȼ��ȡ������Һ�������� | δ��ש��ɫ�������� | ������δˮ�� |
A.AB.BC.CD.D