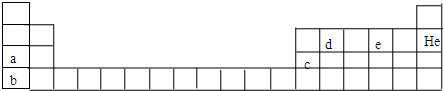
��Ŀ����
Ԫ�����ڱ���ѧϰ��ѧ����Ҫ���ߣ���������������Ϣ���ɡ��±����������ֶ�����Ԫ�ص�ԭ�Ӱ뾶����Ҫ���ϼۣ�Ԫ�ش��� | A | B | C | D | E |
ԭ�Ӱ뾶��nm | 0.16 | 0.143 | 0.102 | 0.089 | 0.074 |
��Ҫ���ϼ� | +2 | +3 | +6��-2 | +2 | -2 |
(1)��Ԫ�ش��ű�����������ڱ��ж�Ӧλ��
(2)C��E�γɻ�����Ϊ______________(�ѧʽ)�����ڱ���ЩԪ�ش��ڡ��Խ������ơ�������д��D�ĵ�����ǿ����Һ��Ӧ�����ӷ���ʽ��______________________�����õ���ʽ��ʾ���Ȼ�����γɹ���____________________________________________________________��
(3)��һ�������Ϸ�Ӧ��ͨʽ���Ա�ʾΪ������+������(1)=������(2)����д����������Ҫ
���4�����������Ϸ�Ӧ�Ļ�ѧ����ʽ��
�����漰��Ԫ�ص�ԭ��������С��30����4�ֵ��ʷ���4����ͬ�塣
_______________________________________��_______________________________________��
_______________________________________��_______________________________________��
(4)MΪ����Ԫ���(Re)�������Ȼ�������һ�ֱ�ŵ��˵���λ��ѧ����1926�귢�ֵ�Ԫ�ء����Ǵ����ԶּƵĺ��ж���Ԫ�صĿ�ʯ�У�ͨ�����ӵĹ���һ��һ�εظ���Ũ�������Ƶ��˽�2mg�Ľ���瑱��ִ���ҵ�ϲ��õķ����ǣ���
��Ԫ��蝹�ԭ������Ϊ_____________���Ԫ�ط�����˼��Ѻ�ʱ�������������ԭ��
____________________________________________________________________��
��д���ִ���ҵ��������ұ������蝹Ļ�ѧ����ʽ�����������ת�Ƶķ������Ŀ��_____________________________________________________________________��
(1)

(2)SO2��SO3 Be+2OH-=![]() +H2�� ����ʽ��
+H2�� ����ʽ��
(3)O2+2NO=2NO2 Cl2+2FeCl2=2FeCl3 Fe+2FeCl3=3FeCl![]() 2CO
2CO
(4)��75 �Ԫ������Ȼ��Ĵ��ڷ�ɢ����ʯ�к����ͣ����ڸ������Ԫ��������Ԫ�ع��棬���Է��롣
![]()

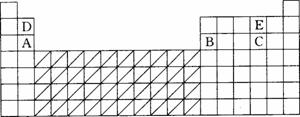
| A�������ң�Ԫ�����ڱ��еĵ�ʮ����ΪVA�� | B��ijIIA��Ԫ�ص�ԭ������Ϊx��������ͬ���ڵ�IIIA��Ԫ�ص�ԭ����������Ϊx+25 | C��VIA��Ԫ�أ���ԭ�Ӱ뾶����Ӧ��̬�⻯����ȶ�����ǿ | D��53��Ԫ��λ�����ڱ��е�5����VIIA�� |
 NH4++OH-
NH4++OH-