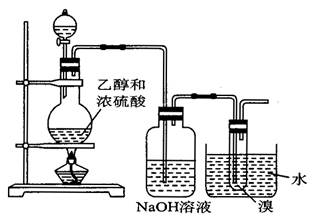
��Ŀ����
(10��)��ѧ��ѧ�м��ֳ������ʵ�ת����ϵ���£�(��ʾ��A�����пɱ��̬������Ӧ���ɵͼ�̬)
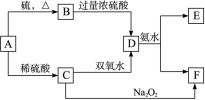
��D��Һ�����ˮ�пɵõ���FΪ��ɢ�ʵĺ��ɫ���塣��ش��������⣺
(1)���ɫ����F����ֱ����С�ķ�Χ��________��
(2)B�Ļ�ѧʽ��________��
(3)д����D����Һ�백ˮ��Ӧ�����ӷ���ʽ��
________________________________________________________________________��
C����Һ��˫��ˮ��Ӧ�����ӷ���ʽ��
________________________________________________________________________��
(4)д������E�������ӵ�ʵ�鷽��������
________________________________________________________________________��

��D��Һ�����ˮ�пɵõ���FΪ��ɢ�ʵĺ��ɫ���塣��ش��������⣺
(1)���ɫ����F����ֱ����С�ķ�Χ��________��
(2)B�Ļ�ѧʽ��________��
(3)д����D����Һ�백ˮ��Ӧ�����ӷ���ʽ��
________________________________________________________________________��
C����Һ��˫��ˮ��Ӧ�����ӷ���ʽ��
________________________________________________________________________��
(4)д������E�������ӵ�ʵ�鷽��������
________________________________________________________________________��
(1)1��100 nm
(2)FeS
(3)Fe3����3NH3��H2O===Fe(OH)3����3NH��2Fe2����H2O2��2H��===2Fe3����2H2O
(4)ȡ����E��Һ��һ�Թ��У���������ŨNaOH��Һ�����Ȳ�����������ʹʪ��ĺ�ɫʯ����ֽ����ɫ����֤����Һ�к�NH?
(2)FeS
(3)Fe3����3NH3��H2O===Fe(OH)3����3NH��2Fe2����H2O2��2H��===2Fe3����2H2O
(4)ȡ����E��Һ��һ�Թ��У���������ŨNaOH��Һ�����Ȳ�����������ʹʪ��ĺ�ɫʯ����ֽ����ɫ����֤����Һ�к�NH?
��FΪFe(OH)3֪AΪFe��BΪFeS��CΪFeSO4��DΪFe2(SO4)3��EΪ(NH4)2SO4��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 5CO2+11H2O+2K2SO4+4MnSO4
5CO2+11H2O+2K2SO4+4MnSO4