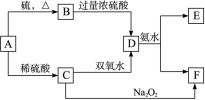
��Ŀ����
( 12�� ) ��ͼ��ʾ��װ�������Ʊ�1��2 -�������� ( ���ƿ�е�NaOH��Һ��Ϊ�����ո���Ӧ������SO2�� ) ����ش���������:
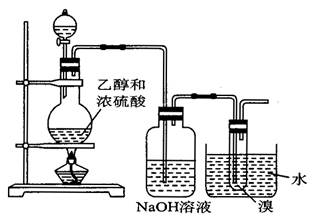
( 1 ) ���Ȼ������170 �� ������Ϊ�� ____________________ ��
( 2 ) ���ƿ��NaOH��Һ������д������SO2��Ӧ�Ļ�ѧ����ʽ ___________________ ��
( 3 ) ʹ��ˮ����ȴ����Ϊ��ʲô��
( 4 ) ��ƿ�еĻ��Һ����ʱ�ȱ���ɫ����ɫ������ _________________________ ��
( 5 ) ��ƿ�з�Щ�״�Ƭ����������ʲô��
( 6 ) ���ɵ�1��2 -��������ͨ�����й������壬Ϊϴȥ������� ______________ ��

( 1 ) ���Ȼ������170 �� ������Ϊ�� ____________________ ��
( 2 ) ���ƿ��NaOH��Һ������д������SO2��Ӧ�Ļ�ѧ����ʽ ___________________ ��
( 3 ) ʹ��ˮ����ȴ����Ϊ��ʲô��
( 4 ) ��ƿ�еĻ��Һ����ʱ�ȱ���ɫ����ɫ������ _________________________ ��
( 5 ) ��ƿ�з�Щ�״�Ƭ����������ʲô��
( 6 ) ���ɵ�1��2 -��������ͨ�����й������壬Ϊϴȥ������� ______________ ��
( 1 ) ����ϩ
? ( 2 ) SO2 + 2NaOH�T�TNa2SO3 + H2O
? ( 3 ) ��ֹ��ӷ�
? ( 4 ) ŨH2SO4ʹ�����Ҵ�����Ϊ̼
? ( 5 ) ��ֹ����
? ( 6 ) NaOH��Һ
? ( 2 ) SO2 + 2NaOH�T�TNa2SO3 + H2O
? ( 3 ) ��ֹ��ӷ�
? ( 4 ) ŨH2SO4ʹ�����Ҵ�����Ϊ̼
? ( 5 ) ��ֹ����
? ( 6 ) NaOH��Һ
�����Ƕ�ʵ��������ϩ�Ŀ��飬ԭ����CH3CH2OH 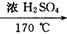 CH2�TCH2�� + H2O�����ڵĸ���Ӧ�Т�ŨH2SO4ʹ�Ҵ���ˮ̼����������2H2SO4 ( Ũ ) + C�T�T2SO2�� + CO2�� + 2H2O��Ӧ����CH3CH2OH + HO��CH2CH3
CH2�TCH2�� + H2O�����ڵĸ���Ӧ�Т�ŨH2SO4ʹ�Ҵ���ˮ̼����������2H2SO4 ( Ũ ) + C�T�T2SO2�� + CO2�� + 2H2O��Ӧ����CH3CH2OH + HO��CH2CH3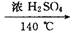 CH3CH2��O��CH2CH3 ( ���� ) + H2O�����Ա�������¶���170 ��,�����������ϩ���ʵ���֤��CH2�TCH2
CH3CH2��O��CH2CH3 ( ���� ) + H2O�����Ա�������¶���170 ��,�����������ϩ���ʵ���֤��CH2�TCH2 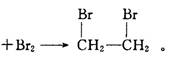
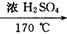 CH2�TCH2�� + H2O�����ڵĸ���Ӧ�Т�ŨH2SO4ʹ�Ҵ���ˮ̼����������2H2SO4 ( Ũ ) + C�T�T2SO2�� + CO2�� + 2H2O��Ӧ����CH3CH2OH + HO��CH2CH3
CH2�TCH2�� + H2O�����ڵĸ���Ӧ�Т�ŨH2SO4ʹ�Ҵ���ˮ̼����������2H2SO4 ( Ũ ) + C�T�T2SO2�� + CO2�� + 2H2O��Ӧ����CH3CH2OH + HO��CH2CH3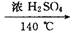 CH3CH2��O��CH2CH3 ( ���� ) + H2O�����Ա�������¶���170 ��,�����������ϩ���ʵ���֤��CH2�TCH2
CH3CH2��O��CH2CH3 ( ���� ) + H2O�����Ա�������¶���170 ��,�����������ϩ���ʵ���֤��CH2�TCH2 

��ϰ��ϵ�д�
�����Ŀ
 Z+W
Z+W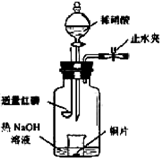
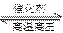 2NH3(g) ��H����92.4 kJ/mol
2NH3(g) ��H����92.4 kJ/mol