��Ŀ����
(10��)��ͼ�������Լ�ƿ��ǩ�ϵ����ݣ�
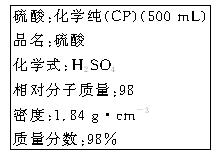
(1)����������ʵ���Ũ��Ϊ________ mol��L��1��
(2)ij��ѧ��ȤС������������ʵ�ʵ��̽��ʱ����Ҫ240 mL 4.6 mol��L��1��ϡH2SO4������ȡ________ mL�ĸ����ᣬ�������������ϡ�͵�ʵ�����Ϊ
________________________________________________________________________��
(3)������4.6 mol��L��1��ϡH2SO4�Ĺ����У����������������Һ���ʵ���Ũ���к�Ӱ��(�ƫ�ߡ���ƫ�͡�����Ӱ�족)
��δ����ȴ���Ƚ���Һע������ƿ�У�________��
������ƿ��1 mol��L��1��ϡH2SO4��ϴ��________��
�۶���ʱ����Һ�������________��

(1)����������ʵ���Ũ��Ϊ________ mol��L��1��
(2)ij��ѧ��ȤС������������ʵ�ʵ��̽��ʱ����Ҫ240 mL 4.6 mol��L��1��ϡH2SO4������ȡ________ mL�ĸ����ᣬ�������������ϡ�͵�ʵ�����Ϊ
________________________________________________________________________��
(3)������4.6 mol��L��1��ϡH2SO4�Ĺ����У����������������Һ���ʵ���Ũ���к�Ӱ��(�ƫ�ߡ���ƫ�͡�����Ӱ�족)
��δ����ȴ���Ƚ���Һע������ƿ�У�________��
������ƿ��1 mol��L��1��ϡH2SO4��ϴ��________��
�۶���ʱ����Һ�������________��
(1)18.4
(2)60����Ũ���������ڻ���ע��ˮ�У��������ò�����������Һ
(3)��ƫ�ߡ���ƫ�ߡ���ƫ��
(2)60����Ũ���������ڻ���ע��ˮ�У��������ò�����������Һ
(3)��ƫ�ߡ���ƫ�ߡ���ƫ��
(1)�����ʵ��������������ʵ���Ũ��ʱ�����ݹ�ʽc�������㣬�������ݿ���ã�c��18.4mol��L��1��
(2)��ϡ��ǰ�����ʵ����ʵ��������֪��18.4mol��L��1��V��4.6mol��L��1��0.24 L����V��0.06 L��
(3)δ����ȴ���Ƚ���Һע������ƿ�У���ȴ�������Һ�����С��������ҺŨ��ƫ�ߣ�����ƿ��1mol��L��1��ϡH2SO4��ϴ�����ʵ����ʵ����������ҺŨ��ƫ�ߣ�����ʱ����Һ���������ˮ�����٣����ƫ�ߡ�
(2)��ϡ��ǰ�����ʵ����ʵ��������֪��18.4mol��L��1��V��4.6mol��L��1��0.24 L����V��0.06 L��
(3)δ����ȴ���Ƚ���Һע������ƿ�У���ȴ�������Һ�����С��������ҺŨ��ƫ�ߣ�����ƿ��1mol��L��1��ϡH2SO4��ϴ�����ʵ����ʵ����������ҺŨ��ƫ�ߣ�����ʱ����Һ���������ˮ�����٣����ƫ�ߡ�

��ϰ��ϵ�д�
 �ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
�ִʾ�ƪ��ͬ�����Ĵ��ϵ�д� �߽�������ϵ�д�
�߽�������ϵ�д�
�����Ŀ
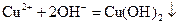 ����
����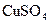 ��Һ���백ˮ�У����������ɫ����
��Һ���백ˮ�У����������ɫ����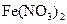 ��Һ�д���ƽ�⣺
��Һ�д���ƽ�⣺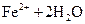
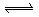
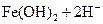 ����������ϡ�������Һdz��ɫ�����
����������ϡ�������Һdz��ɫ�����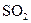 ��Ư���ԣ���
��Ư���ԣ���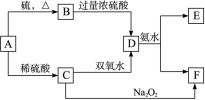


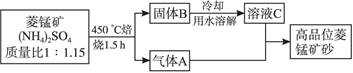
 2KMnO4+2KOH+H2��
2KMnO4+2KOH+H2��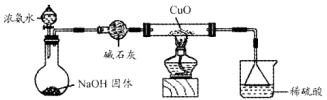
 N2+3Cu+3H2O��
N2+3Cu+3H2O��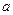 mol������ձ��е���Һ��ʹ��̪��졣�����ݡ���ѧʵ����ƻ���Ҫ������Ʊ������������Һ�ķ����������ʵ�鷽����
mol������ձ��е���Һ��ʹ��̪��졣�����ݡ���ѧʵ����ƻ���Ҫ������Ʊ������������Һ�ķ����������ʵ�鷽����