��Ŀ����
����ֲ���纣���������к��зḻ�ĵ�Ԫ�أ���Ԫ���Ե�������ʽ���ڡ�ʵ���ҴӺ�������ȡ����������£�
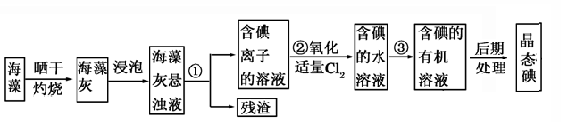
��1��ָ����ȡ��Ĺ������й�ʵ����������ƣ�
��Ϊ ����Ϊ �����̢����йط�Ӧ�����ӷ���ʽ�� ��
��2����ȡ��Ĺ����пɹ�ѡ����л��ܼ���( )
��3��Ϊʹ������е�����ת��Ϊ����л���Һ��ʵ���������ձ����������� ����ƿ���ƾ��ơ����ܡ�Բ����ƿ��ʯ�������Լ���Ҫ�ļг�װ�á���Ʒ����ȱ�ٵIJ��������� ��
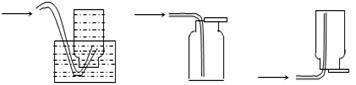
��4���Ӻ�����л��ܼ�����ȡ��ͻ����л��ܼ�������Ҫ��������ָ����ͼ��ʾ��ʵ��װ���еĴ���֮���� �� ���� ��
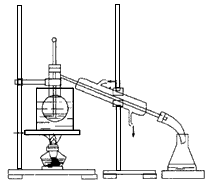
��5�����������������ʱ��ʹ��ˮԡ���ȵ�ԭ���� �����̬���� ��ۼ���

��1��ָ����ȡ��Ĺ������й�ʵ����������ƣ�
��Ϊ ����Ϊ �����̢����йط�Ӧ�����ӷ���ʽ�� ��
��2����ȡ��Ĺ����пɹ�ѡ����л��ܼ���( )
| A���ױ����ƾ� | B�����Ȼ�̼���� | C�����͡����� | D�����͡����� |
��4���Ӻ�����л��ܼ�����ȡ��ͻ����л��ܼ�������Ҫ��������ָ����ͼ��ʾ��ʵ��װ���еĴ���֮���� �� ���� ��

��5�����������������ʱ��ʹ��ˮԡ���ȵ�ԭ���� �����̬���� ��ۼ���
��1������ ��ȡ Cl2+2I��=I2+2Cl��
��2��B ��3����Һ©����©��
��4�����¶ȼƲ嵽��Һ���� �������ܽ���ˮ�ķ���ߵ�
��5��ʹ������ƿ�������ȣ����Ƽ����¶Ȳ�Ҫ���� ������ƿ
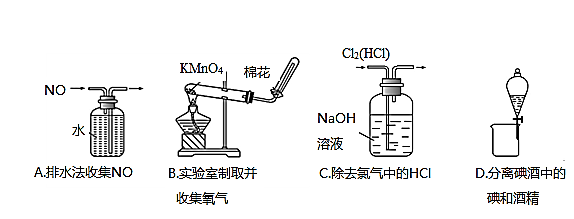
���������������һ���ۺ�ʵ�鿼���⣬������Ǻ����еⵥ�ʵ���ȡ����Ҫ�Ӻ��������ճɻң�������ˮ���õ����ӽ��뵽��Һ�У�Ȼ����ǿ�������ѵ����������ɶ��ⵥ�ʣ��������Ȼ�̼�ѵⵥ�ʽ�����ȡ�����ѵ����ȡҺ���з��룬���������Ȼ�̼ʱ��һ��Ҫ������ķ�������Ϊ���Ȼ�̼�ķе�ͣ���˵�������������ƿ�С�

��ϰ��ϵ�д�
�����Ŀ

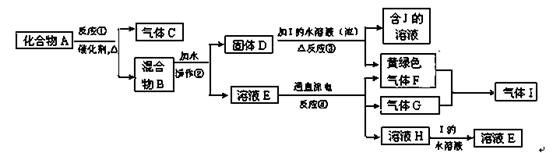
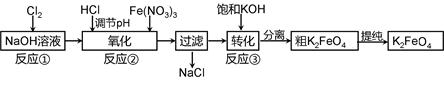
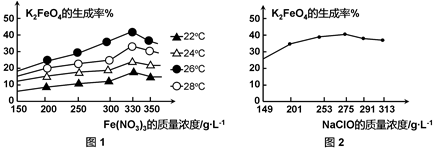
 4Fe(OH)3 + 8OH- + 3O2���ڡ��ᴿ��K2FeO4�в����ؽᾧ��ϴ�ӡ����º�ɵķ�������ϴ�Ӽ����ѡ�� ��Һ������ţ���
4Fe(OH)3 + 8OH- + 3O2���ڡ��ᴿ��K2FeO4�в����ؽᾧ��ϴ�ӡ����º�ɵķ�������ϴ�Ӽ����ѡ�� ��Һ������ţ���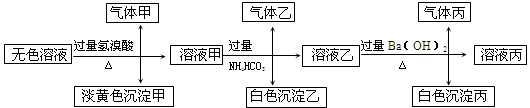
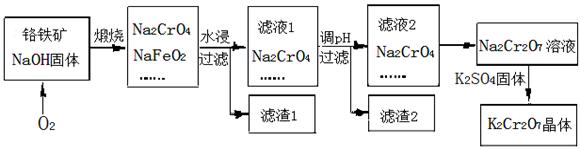
 2CrO42�� + 2H+
2CrO42�� + 2H+