��Ŀ����
����Ŀ���ǻ�����ҽҩ��������״���������μ���Р̵�����������Ҳ����ʳƷ���Ӽ����й��������ҵ����ˮ����(N2H4��H2O)��ԭ����ȡ�⻯�ƹ��壬���Ʊ�������ͼ��ʾ��
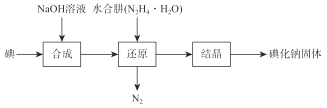
��֪��N2H4��H2O��100�����Ϸֽ⡣
(1)�ںϳ�NaI�Ĺ����У����ܻ��е�������_______������������Ҫ�ʵ�����NaOH��Ŀ����________��
(2)�ڻ�ԭ�����У�Ϊ�˷�ֹˮ����(N2H4��H2O)���·ֽ�����Ӧ�¶ȿ�����60~70�棬�¶�Ҳ���ܹ��ͣ���ԭ����___________����ҵ��Ҳ���������ƻ���м��ԭ�������Ʊ��⻯�ƣ���ˮ���»�ԭ���ƵõIJ�Ʒ���ȸ��ߣ���ԭ����_____________________��
(3)�����һ����ʵ�������黹ԭҺ���Ƿ���IO3-��__________________��(�ɹ�ѡ����Լ���ϡ���ᡢ������Һ��FeCl3��Һ)
(4)�ⶨ��Ʒ��NaI������ʵ�鲽�����£�
a.��ȡ4.000 g��Ʒ���ܽ⣬��250 mL����ƿ�ж��ݣ�
b.��ȡ25.00 mL����Һ����ƿ�У�Ȼ�����������FeCl3��Һ����ַ�Ӧ���ټ���A��Һ��ָʾ����
c.��0.1000mol��L-1��Na2S2O3��Һ�ζ����յ�(������Ӧ����ʽΪ��2Na2S2O3+I2=Na2S4O6+2NaI)���ظ��ⶨ3�Σ����õ�������������ʾ��
�ⶨ��� | �������/ml | ʢװ���ζ��ܵ�������/ml | ʢװ���ζ��ܵ��յ����/ml |
1 | 25.00 | 0.06 | 24.04 |
2 | 25.00 | 0.02 | 24.02 |
3 | 25.00 | 0.12 | 24.14 |
���ڵζ������У�Na2S2O3��ҺӦ��___________(������)���������A����Ϊ____________(������)��
���ζ��յ�۲쵽������Ϊ_______________________��
������Ʒ��NaI�ĺ���Ϊ_______________________��
�����������������NaI�ĺ���ƫ��(���Բⶨ�����е����)������ܵ�ԭ����_________________��
(5)�⻯�ƹ���ı��淽����_______________________��
���𰸡� NaIO3 ��ʹ��Ӧ�������﷽���ƶ� �¶ȹ��ͣ����ͻ�ѧ��Ӧ���� N2H4��H2O��������IJ���ΪN2��H2O������������ ȡ������ԭҺ�����������Һ����ϡ�����ữ������Һ����ɫ��˵����ԭҺ�к���IO3-������Һ��������˵����ԭҺ�в���IO3- ��ʽ�ζ��� ���� ��Һ��ɫ��ȥ���Ұ�����ڲ��ָ� 90% ����NaI��������O2���� ����ɫƿ�ڹ⡢�ܷⱣ��
����������1������������Ʒ�����Ӧ��3I2+6NaOH=5NaI+NaIO3+3H2O����NaI�п��ܻ���NaIO3��Ϊ��ʹ��Ӧ�������﷽���ƶ�������������Ҫ�ʵ�����NaOH����2���ڻ�ԭ�����У�Ϊ�˷�ֹˮ���£�N2H4H2O�����·ֽ⣬��Ӧ�¶ȿ�����60��70�棬�¶�Ҳ���ܹ��ͣ��¶ȹ��ͣ����ͻ�ѧ��Ӧ���ʣ�N2H4H2O����������ΪN2��H2O������Na2S��Feм��ԭ��õ��������ʣ�ˮ���»�ԭ���ƵõIJ�Ʒ���ȸ��ߣ���3�������������������Һ���ܰѻ�ԭΪ���ʵ⣬��˼��黹ԭҺ���Ƿ���IO3-��ʵ�鷽����ȡ������ԭҺ�����������Һ����ϡ�����ữ������Һ����ɫ��˵����ˮ�к���IO3-������Һ��������˵����ˮ�в���IO3-����4����Na2S2O3Ϊǿ�������Σ���Ϊ������������ˮ�⣬Na2S2O3��Һ�������ԣ����Եζ�ʱNa2S2O3��ҺӦ���ڼ��Եζ����У���Ϊ�����������ɫ�����Լ���ⵥ���õ�����Һ��ָʾ�����ڼ���A����Ϊ������Һ���ζ��յ�۲쵽������Ϊ��Һ����ɫ��ɫ���Ұ�����ڲ���ɫ���۸��ݱ���3������Na2S2O3�������ȡ��ƽ��ֵΪ24.00mL����2Fe3++2I-��2Fe2++I2��2Na2S2O3+I2��Na2S4O6+2NaI����2I-��2Na2S2O3������Ʒ��NaI�ĺ���Ϊ��(24��103��0.1000��150��250/25)/4.000��100%=90%�� �ܲ���NaI��������O2�������������������NaI�ĺ���ƫ�ͣ���5��NaI�ױ�������O2�������⻯�ƹ���Ӧ�ܹ��ܷⱣ�棬������ɫƿ�ڹ⡢�ܷⱣ�档

