��Ŀ����
����Ŀ����14�֣��״���һ����Ҫ�Ŀ�������Դ��
��1����֪2CH4(g)+O2(g)=2CO(g)+4H2(g) ��H =a KJ/mol
CO(g)+2H2(g)=CH3OH(g) ��H =b KJ/mol
��д����CH4��O2��ȡ�״����Ȼ�ѧ����ʽ�� ��
��2��������ͨ�����з�Ӧ�Ʊ��״���CO(g)+2H2(g) ![]() CH3OH(g)��
CH3OH(g)��
��ͼ�Ƿ�ӦʱCO��CH3OH(g)��Ũ����ʱ��ı仯������ӷ�Ӧ��ʼ����ƽ�⣬��H2��ʾƽ����Ӧ���ʦ�(H2)= _��
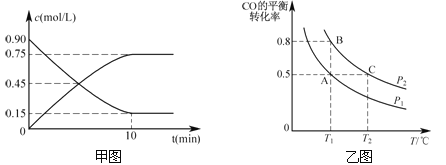
��3����һ�ݻ��ɱ���ܱ������г���10 mol CO��20 mol H2��CO��ƽ��ת�������¶ȣ�Tѹǿ��P���ı仯����ͼ��ʾ��
������˵�����жϸ÷�Ӧ�ﵽ��ѧƽ��״̬����_______��������ĸ��
A��H2���������ʵ���CH3OH���������ʵ�2��
B��H2������������ٸı�
C����ϵ��H2��ת���ʺ�CO��ת�������
D����ϵ�������ƽ��Ħ���������ٸı�
�ڱȽ�A��B����ѹǿ��СPA________PB�����������=������
�����ﵽ��ѧƽ��״̬Aʱ�����������Ϊ20 L�������Ӧ��ʼʱ�Գ���10 molCO��20 molH2������ƽ��״̬Bʱ���������V(B)= L��
��4���Լ״�Ϊȼ�ϣ�����Ϊ��������KOH��ҺΪ�������Һ�����Ƴ�ȼ�ϵ�أ��缫����Ϊ���Ե缫����
����KOH��Һ��������д������ܷ�Ӧ�����ӷ���ʽ��___________________��
�����������Һ��KOH�����ʵ���Ϊ0.8 mol������0.5 mol�״����뷴Ӧʱ���������Һ�и������ӵ����ʵ���Ũ���ɴ�С��˳���� ��
���𰸡� (1) 2CH4(g)+O2(g)=2CH3OH(g) ��H=(a+2b)kJ/mol (2��) (2) 0.15mol/(L��min) (2��)
(3) ��BD (2��) �� ��(2��) �� 14L (2��) (4) �� 2CH3OH+3O2+4OH-=2CO32-+6H2O(2��)
��C(K+)>C(CO32-)>C(HCO3-)>C(OH-)>C(H+)(2��)
��������
���������(1) �������ķ�Ӧ�ֱ��Ϊ���١��ڣ����ݸ�˹����������+�ڡ�2�ɵ�CH4��O2��ȡ�״����Ȼ�ѧ����ʽ��2CH4(g)+O2(g)=2CH3OH(g) ��H=(a+2b)kJ/mol��(2) ��ͼ���Կ���CO��Ũ�ȼ�С��0.75mol/L�����ݷ�Ӧ�Ļ�ѧ��������H2��Ũ�ȸı���1.5mol/L����Ӧ�ﵽƽ���ʱ��Ϊ10min�����(H2)= 1.5mol/L��10min=0.15mol/(L��min)��(3) �ٻ�ѧ��Ӧ����֮�ȵ��ڻ�ѧ������֮�ȣ��������κ�ʱ��H2���������ʵ���CH3OH���������ʵ�2����A����H2������������ٸı�˵����ϵ�и����ʵ�Ũ�Ȳ��ٸı䣬��Ӧ�ﵽ��ƽ�⣬B�ԣ�H2��ת���ʺ�CO��ת������Ȳ���˵�����ʵ�Ũ�Ȳ��ٸı���ͬ�����ʵ����淴Ӧ������ȣ�C������Ӧ���������ʵ����ı�ķ�Ӧ�������Ӧû�дﵽƽ�⣬��������ʵ����ᷢ���ı䣬ƽ��Ħ������Ҳ��ı䣬��ƽ��Ħ���������ٸı䣬˵����������ɺ�����������ȣ���Ӧ������ƽ�⣬D�ԡ��ڴ�ͼ���Կ���B��CO��ת���ʽϴ��ݷ�Ӧ���ص㣬ѹǿ����ƽ���������ƶ�����B���ת���ʽϴ�˵��B���ѹǿ��PA ��PB ����A����CO��ת����Ϊ0.5��10 mol CO��20 mol H2��Ӧ�����������ʵ���Ϊ20mol����B��CO��ת����Ϊ0.8��10 mol CO��20 mol H2��Ӧ������������ʵ���Ϊ14mol����B������ΪVL����20mol�U14 mol=20L�UV��V=14L����4���������ڼ��Ե������Һ�У�������CO2��Ҫ��Ӧ����Ӧ�ķ���ʽΪ��2CH3OH+3O2+4OH-=2CO32-+6H2O���ڸ���KOH��CO2�����ʵ�����֪���ɵ�CO2������Ӧ��CO2+2OH���TCO32����CO32��+ CO2+H2O�THCO3��,0.8mol��KOH��Ӧ��ʱ����0.4mol��CO2������0.4mol��CO32����ʣ���0.1mol��CO2��CO32����Ӧ����0.1mol��HCO3��������Һ����0.3mol��K2CO3��0.1mol��KHCO3��������Ũ�ȴ�С˳��Ϊ��C(K+)>C(CO32-)>C(HCO3-)>C(OH-)>C(H+)

����Ŀ��������������������Ҫ��ʩ�л�������ʱ��ͨ���ơ�������ҵͣҵ�����ﳾ��Ⱦ���Ƶȡ�
(l) PM2.5�ǻ������ż�������������Ҫָ�ꡣ��ijPM2.5����������ˮ�����Ƴɴ�����������������������ӵ������ƽ��Ũ�����±���
�������� | Na+ | NH4+ | SO42- | NO3- |
Ũ��/(mol/L) | 2.0��l0-6 | 2.8��10-5 | 3.5��10-5 | 6.0��l0-5 |
��������pHΪ____________��
(2����������Ҫ�ɷ�֮һ����������β���ĵ�������о�����CH4������������β���е����������Ⱦ��
�� CH4(g)+2O2(g)=CO2(g)+2H2O(l) ��H��-889.6KJ/mol
�� N2(g)+2O2(g)=2NO2��g����H=��67.2KJ/mol
�� 2NO2(g) ![]() N2O4(g) ��H��-56.9KJ/mol
N2O4(g) ��H��-56.9KJ/mol
д�������������ԭN2O4���������ȶ��ĵ������塢������̼�����Һ̬ˮ���Ȼ�ѧ����ʽ��_____��
(3��һ�������£���CO ��H2�ϳ������ԴCH3OH�����Ȼ�ѧ����ʽΪCO(g) + 2H2(g) ![]() CH3OH (g) ��H,CO��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��
CH3OH (g) ��H,CO��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��
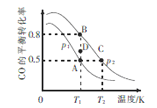
���ÿ��淴Ӧ����H_____0������>����<����=������A��B, C�����Ӧ��ƽ�ⳣ��KA��KB��KC�Ĵ�С��ϵ��_________��ѹǿ��p1_______p2������>����<����=��������T1�����£���D�㵽B������У������淴Ӧ����֮��Ĺ�ϵ��v��________v�棨����>����<����=������
�����ں��º��������½���������Ӧ���ܱ�ʾ�ÿ��淴Ӧ�ﵽƽ��״̬����__________������ĸ��
A. CO������������ֲ���
B�������ڻ��������ܶȱ��ֲ���
C�������ڻ�������ƽ��Ħ���������ֲ���
D����λʱ��������CO��Ũ�ȵ�������CH3OH��Ũ��
�����ѹ�ܱ������г���2mol CO��4mol H2����p2��T2�����´ﵽƽ��״̬C�㣬��ʱ�����ݻ�Ϊ2L�����ڸ������·�Ӧ��ƽ�ֳ���KΪ______________��
����Ŀ���ǻ�����ҽҩ��������״���������μ���Р̵�����������Ҳ����ʳƷ���Ӽ����й��������ҵ����ˮ����(N2H4��H2O)��ԭ����ȡ�⻯�ƹ��壬���Ʊ�������ͼ��ʾ��
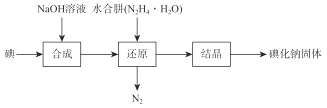
��֪��N2H4��H2O��100�����Ϸֽ⡣
(1)�ںϳ�NaI�Ĺ����У����ܻ��е�������_______������������Ҫ�ʵ�����NaOH��Ŀ����________��
(2)�ڻ�ԭ�����У�Ϊ�˷�ֹˮ����(N2H4��H2O)���·ֽ�����Ӧ�¶ȿ�����60~70�棬�¶�Ҳ���ܹ��ͣ���ԭ����___________����ҵ��Ҳ���������ƻ���м��ԭ�������Ʊ��⻯�ƣ���ˮ���»�ԭ���ƵõIJ�Ʒ���ȸ��ߣ���ԭ����_____________________��
(3)�����һ����ʵ�������黹ԭҺ���Ƿ���IO3-��__________________��(�ɹ�ѡ����Լ���ϡ���ᡢ������Һ��FeCl3��Һ)
(4)�ⶨ��Ʒ��NaI������ʵ�鲽�����£�
a.��ȡ4.000 g��Ʒ���ܽ⣬��250 mL����ƿ�ж��ݣ�
b.��ȡ25.00 mL����Һ����ƿ�У�Ȼ�����������FeCl3��Һ����ַ�Ӧ���ټ���A��Һ��ָʾ����
c.��0.1000mol��L-1��Na2S2O3��Һ�ζ����յ�(������Ӧ����ʽΪ��2Na2S2O3+I2=Na2S4O6+2NaI)���ظ��ⶨ3�Σ����õ�������������ʾ��
�ⶨ��� | �������/ml | ʢװ���ζ��ܵ�������/ml | ʢװ���ζ��ܵ��յ����/ml |
1 | 25.00 | 0.06 | 24.04 |
2 | 25.00 | 0.02 | 24.02 |
3 | 25.00 | 0.12 | 24.14 |
���ڵζ������У�Na2S2O3��ҺӦ��___________(������)���������A����Ϊ____________(������)��
���ζ��յ�۲쵽������Ϊ_______________________��
������Ʒ��NaI�ĺ���Ϊ_______________________��
�����������������NaI�ĺ���ƫ��(���Բⶨ�����е����)������ܵ�ԭ����_________________��
(5)�⻯�ƹ���ı��淽����_______________________��