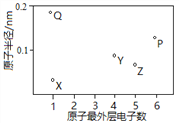
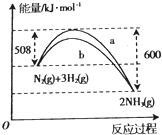
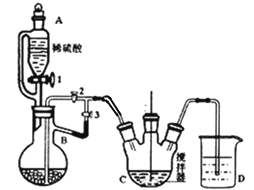
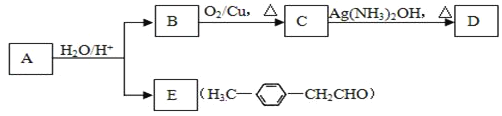
��Ŀ����
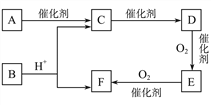
����Ŀ����֪:DΪ��;E������̼Ԫ������Ԫ�ص�����֮��6��1,��Է�������Ϊ44,��ȼ�ղ���ֻ��CO2��H2O��A�����ʽ��F��ͬ,���ܷ���������Ӧ,���ɵ���ˮ��õ���
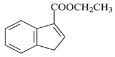
��1��A�Ľṹ��ʽΪ__________________��
��2��д��D��E�Ļ�ѧ����ʽ:_______________________��
��3������˵����ȷ����____��
A.�л���F��ʹʯ����Һ���
B.�����Ƶ�������ͭ�������л���C��E��F��ˮ��Һ
C.�����ʵ�����C��D�ֱ���ȫȼ�����������������
D.���ñ���̼������Һ��ȥ�л���B�л��е�����C��F
E. B��ͬ���칹�����ܷ���������Ӧ�������2��
���𰸡� CH2OH(CHOH)4CHO 2CH2![]() CH2+O2
CH2+O2![]() 2CH3CHO ACDE
2CH3CHO ACDE
��������A�ܷ���������Ӧ�����ɵ���ˮ��õ���˵��AΪ�����ǣ��������ڴ�������������CΪ�Ҵ���E������̼Ԫ������Ԫ�ص�����֮��6��1��˵��E������C��Hԭ�Ӹ�����Ϊ1:2����Է�������Ϊ44����ȼ�ղ���ֻ��CO2��H2O����EΪCH3CHO��DΪ��������C��E��֪��DΪ��ϩ��F�����ʽ����������ͬ��������ȩ�������õ���˵��FΪ���ᣬB����������������������Ҵ�����BΪ����������
(1). AΪ�����ǣ���ṹ��ʽΪCH2OH(CHOH)4CHO���ʴ�Ϊ��CH2OH(CHOH)4CHO��
(2). ��ϩ������������ȩ�Ļ�ѧ����ʽΪ��2CH2![]() CH2+O2
CH2+O2![]() 2CH3CHO���ʴ�Ϊ��2CH2
2CH3CHO���ʴ�Ϊ��2CH2![]() CH2+O2
CH2+O2![]() 2CH3CHO��
2CH3CHO��
(3). A. �л���FΪ���ᣬ�������ԣ���ʹʯ����Һ��죬��A��ȷ��B. CΪ�Ҵ���EΪ��ȩ��FΪ�����������Ҵ�������ˮ�����ֲ㣬��ȩ������������ͭ��Ӧ����ש��ɫ��������������������ˮ�������ֲ㣬�ϲ�Ϊ��״Һ�壬���������ͬ�����Կ������Ƶ�������ͭ���֣���B����C. 1mol�Ҵ���ȫȼ������3mol������1mol��ϩ��ȫȼ������3mol������������ʵ������Ҵ�����ϩ�ֱ���ȫȼ���������������������C��ȷ��D. ����̼������Һ�����ܽ��Ҵ����������������������������ܽ�ȣ����Կ��ñ���̼������Һ��ȥ���������л��е������Ҵ������ᣬ��D��ȷ��E. ����������ͬ���칹�����ܷ���������Ӧ�����������У�HCOOCH2CH2CH3��HCOOCH(CH3)2����2�֣���E��ȷ����ѡ��ACDE��

 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�