��Ŀ����
����Ŀ������֪��Ӧ��R��CH��CH��O��R��(����ϩ����) ![]() R��CH2CHO + R��OH
R��CH2CHO + R��OH
����ϩ����A����Է�������Mr(A)=162��ˮ�����B����Է�������Mr(B)=46����A��صĻ�ѧ��Ӧ���£���ش��������⣺
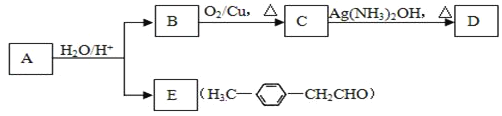
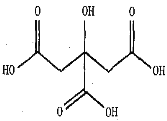
(1)A�Ľṹ��ʽΪ______________________���÷�������_________�ֻ�������ԭ�ӡ�
(2)B��������__________��д��B��C��Ӧ�Ļ�ѧ����ʽ��________________________��
(3)д��C �� D��Ӧ�Ļ�ѧ����ʽ��_______________________________________��
(4)д��ͬʱ��������������E������ͬ���칹��Ľṹ��ʽ�������ڷ���ȩ �ڱ����������ֲ�ͬ��������ԭ��_________________________��
II����֪�� ![]() +ClCH2CH3
+ClCH2CH3 +HCl
+HCl
(5)��д����![]() ��B�ͱ���Ϊԭ�ϣ��ϳ�
��B�ͱ���Ϊԭ�ϣ��ϳ�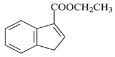 ������ͼ(ע�����Լ�����)____________________��
������ͼ(ע�����Լ�����)____________________��![]()
![]()
���𰸡�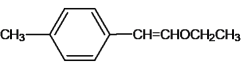 7 �Ҵ� 2CH3CH2OH+O2
7 �Ҵ� 2CH3CH2OH+O2![]() 2CH3CHO+2H2O CH3CHO+2[Ag(NH3)2]OH
2CH3CHO+2H2O CH3CHO+2[Ag(NH3)2]OH![]() CH3COONH4+2Ag+3NH3��+ H2O
CH3COONH4+2Ag+3NH3��+ H2O 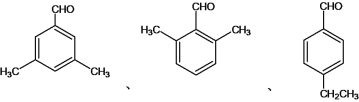
��������
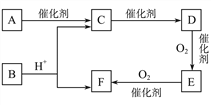
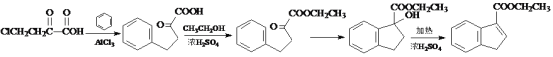
AΪ����ϩ���ѣ�������Ϣ�����Ի����£�����ȩ�ʹ���EΪȩ��BΪ��������B����������ȩ��ȩ�����������ᣬBΪ���������С�CH2OH�Ľṹ����������Է���������BΪ�Ҵ���
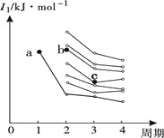
(1) AΪ����ϩ���ѣ�������Ϣ�����Ի����£�����ȩ�ʹ���EΪ�Լ�����ȩ��BΪ�Ҵ���������֪��Ϣ��A�Ľṹ��ʽΪ ���÷����ϵĵ�Ч����ͼ����ʾ��
���÷����ϵĵ�Ч����ͼ����ʾ��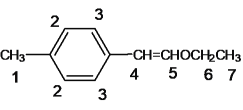 ����7�֣�
����7�֣�
(2) AΪ����ϩ���ѣ�������Ϣ�����Ի����£�����ȩ�ʹ��� BΪ��������B����������ȩ��ȩ�����������ᣬBΪ���������С�CH2OH�Ľṹ����������Է�������Ϊ46��BΪ�Ҵ���B����C�ķ�ӦΪ���Ĵ���������ѧ����ʽΪ2CH3CH2OH+O2![]() 2CH3CHO+2H2O��
2CH3CHO+2H2O��
(3)CΪ��ȩ����������Һ�õ�����泥���ѧ����ʽΪCH3CHO+2[Ag(NH3)2]OH![]() CH3COONH4+2Ag+3NH3��+ H2O��
CH3COONH4+2Ag+3NH3��+ H2O��
(4)�����ڷ���ȩ������������CHO������������2�ֲ�ͬ��������ԭ�ӣ���һ���������ڶ�λ������2����CH3���ڶԳ�λ�á��� ��
��
(5) ![]() ��B�Ҵ����Է���������Ӧ�õ������е�������������֪��
��B�Ҵ����Է���������Ӧ�õ������е�������������֪��![]() �뱽����ȡ����Ӧ���õ�������̼̼˫�����ô�����ȥ��Ӧ�õ�������ͼΪ��
�뱽����ȡ����Ӧ���õ�������̼̼˫�����ô�����ȥ��Ӧ�õ�������ͼΪ�� ��
��

����Ŀ������ϩ�Ǻϳ������ᡢ����ͪ�����ӵȵ���Ҫԭ�ϣ�Ҳ������ʯ����ȡ����������ֵ�����ȶ�����
�Ʊ�����ϩ�ķ�Ӧԭ��������Ӧ![]() ������Ӧ
������Ӧ![]()
�Ʊ�����ϩ��ʵ��װ��ͼ���£�(�г�װ�ü�����װ����ʡ��)
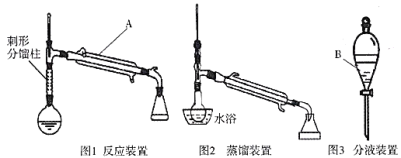
��Ӧ��ʵ����������ͼ��ʾ��

������ʷе㡢�ܶȡ��ܽ������£�
�е�/�� | �ܶ�/( | ˮ���ܽ��� | |
������ | 161 | 0.962 4 | ������ˮ |
����ϩ | 83 | 0.811 | ������ˮ |
85% | 1.69 | ������ˮ | |
����ϩ��ˮ�γɵĹ�����(��ˮ10%) | 70.8 | ||
��������ˮ�γɵĹ�����(��ˮ80%) | 97.8 |
�ش��������⣺
(1)ʵ���в���Ũ���ᣬ����85%![]() ��Һ��˵������________________(д��һ������)��
��Һ��˵������________________(д��һ������)��
(2)��ֲ��ﻷ��ϩ�м���ʳ��ʹˮ�㱥�͵�Ŀ����_____________��ˮԡ����ǰ������ˮ�Ȼ��Ƶ�Ŀ����___________________________.
(3)����A��B�����Ʒֱ���________________________��
(4)�����ᴿʱ�õ�ˮԡ���ȵķ��������ŵ���______________(д����������)��
(5)��������Ҫ��ʵ��ķ�Ӧ�¶�Ӧ������90������ԭ����_____________________��
(6)