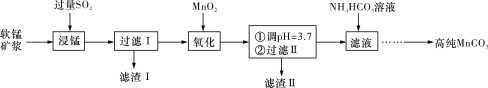
��Ŀ����
����Ŀ������ѧ����ѡ��3�����ʵĽṹ�����ʣ�
���С�����������͡�δ��������֮�ƣ��Ѽ��仯�����Ӧ����Խ��Խ�ܵ����ǵĹ�ע��
��1����̬��ԭ����Χ���ӵĹ������ʽΪ___________������ͬ���ڵ�Ԫ���У���̬ԭ�ӵ�δ�ɶԵ�����������ͬ����___�֡�
��2�������ѵ��۵㡢Ӳ�Ⱦ��������ܵ�ԭ����____��
��3��TiCl4���Ȼ�����ȡ�ѵ��м���TiCl4��SiCl4�ڳ����¶���Һ�壬���ӽṹ��ͬ����������ķ�������TiCl4��SiCl4�Ļ����Ȼ�õ������__________ (�ѧʽ)��

��4������Ľṹ����M�ܴ���ϩ����ϩ������ϩ�ȵľۺϣ���ṹ��ͼ��ʾ��
����ɸ����ʵ�Ԫ���У��縺��������__________ (��Ԫ������)��
��M��̼ԭ�ӵ��ӻ���ʽ��___________�֡�
��M�в���__________ (����ĸ����)��
a���м� ����b���Ҽ� c����λ��
d����� ����e�����Ӽ�

��5���ٽ����Ѿ�����ԭ�Ӳ��������������ܶѻ���������ԭ�ӵ���λ��Ϊ_____��
������ԭ�ӵ�ֱ��Ϊd cm����NA��ʾ�����ӵ�������ֵ����ԭ�ӵ�Ħ������ΪM g��mol��1�������ѵ��ܶ�Ϊ________g��cm��3��
�۽����Ѿ����������ɸ����������϶����ͼ��a��b��c��d�ĸ���ԭ���γ�һ���������壬���ڲ�Ϊ���������϶�������������ԭ�ӡ������������е����������϶�ж������ԭ�ӣ���ô�γɵ��⻯�ѵĻ�ѧʽΪ__________��
���𰸡�![]() 3 Tiԭ�ӵļ۵�������Al������������ǿ SiCl4 �� 2 de 12
3 Tiԭ�ӵļ۵�������Al������������ǿ SiCl4 �� 2 de 12 ![]() TiH2
TiH2
��������
��1������Tiԭ����Χ�����Ų�ʽ3d24s2��������Χ���ӵĹ������ʽ������Tiԭ��δ�ɶԵ�����Ϊ2����4����Ԫ���У���̬ԭ�ӵ�δ�ɶԵ���������ͬ����Ni��3d84s2����Ge��4s24p2����Se��4s24p4����
��2��������ͬΪ�������壬���������Ӳ����Ҫ�ɽ�������������Ӳ�ȱ������ԭ����Tiԭ�ӵļ۵�������Al�࣬��������ǿ��
��3����TiCl4��SiCl4�ڳ����¶���Һ�壬��֪�����߾����ڷ��Ӿ����ҷ��ӽṹ��ͬ�����Ӽ���������Ӱ�쾧���������ʵ���Ҫ���أ���Է�������Խ����Ӽ�������Խ��
��4������ɸ����ʵ�Ԫ���У��縺������������
��̼ԭ�ӵ��ӻ���ʽ��sp2��sp3���֣�
���ڰ���ķ��ӽṹ�У�C-C��C-H��C-Oԭ���д��ڦҼ������д����Ŵ�м���Ti��O�������λ������������������Ӽ���
��5����Ti�������������������ܶѻ����Դ˷���Tiԭ����λ����
�ڸ���![]() =
=![]() ���㾧���ѵ��ܶȣ�
���㾧���ѵ��ܶȣ�
�۸��������Ϣ�����Tiԭ�Ӻ�Hԭ�Ӹ����ȣ�ȷ����ѧʽ��
��1��Ti��ԭ������Ϊ22����̬Tiԭ�Ӻ�����Ų�Ϊ1s22s22p63s23p63d24s2����Χ���ӵĹ������ʽΪ![]() ����δ�ɶԵ�����Ϊ2����̬��ԭ�Ӻ�������Ų�������ܼ��ķ�����3d����4����Ԫ���У���̬ԭ�ӵ�δ�ɶԵ���������ͬ����Ni��3d84s2����Ge��4s24p2����Se��4s24p4��3�֡�
����δ�ɶԵ�����Ϊ2����̬��ԭ�Ӻ�������Ų�������ܼ��ķ�����3d����4����Ԫ���У���̬ԭ�ӵ�δ�ɶԵ���������ͬ����Ni��3d84s2����Ge��4s24p2����Se��4s24p4��3�֡�
�ʴ�Ϊ��![]() ��3��
��3��
��2��������ͬΪ�������壬���������Ӳ����Ҫ�ɽ�������������Ӳ�ȱ������ԭ����Tiԭ�ӵļ۵�������Al�࣬��������ǿ����Ti��ԭ�ӻ��ȱ�Al��������ǿ�����������𰸣���
�ʴ�Ϊ��Tiԭ�ӵļ۵�������Al�࣬��������ǿ��
��3����TiCl4��SiCl4�ڳ����¶���Һ�壬��֪�����߾����ڷ��Ӿ����ҷ��ӽṹ��ͬ�����Ӽ���������Ӱ�쾧���������ʵ���Ҫ���أ���Է�������Խ����Ӽ�������Խ�����ԣ�TiCl4��SiCl4�е�ߡ���������ķ�������TiCl4��SiCl4�Ļ����Ȼ�õ������SiCl4��
�ʴ�Ϊ��SiCl4��
��4������ɸ����ʵ�Ԫ���У��縺������������
��̼ԭ�ӵ��ӻ���ʽ��sp2��sp3���֣�
���ڰ���ķ��ӽṹ�У�C-C��C-H��C-Oԭ���д��ڦҼ������д����Ŵ�м���Ti��O�������λ������������������Ӽ�����ѡd��e��
�ʴ�Ϊ������2��de��
��5���پ�����Tiԭ��λ�����ġ������ϣ����������������ܶѻ����Զ���Tiԭ���о�����֮���ڵ�ԭ�Ӵ������ģ�ÿ������Ϊ8�ʾ������ã�ÿ������Ϊ2���������У���Tiԭ����λ��Ϊ![]() =12��
=12��
�ھ�����Tiԭ����ĿΪ8��![]() +6��
+6��![]() =4����������Ϊ4��
=4����������Ϊ4��![]() g��
g��
��Tiԭ�Ӱ뾶Ϊdcm�������ⳤ=4d cm��![]() =2
=2![]() d cm�����������2
d cm���������Ϊ��2![]() dcm��3�����ܶ�=4��
dcm��3�����ܶ�=4��![]() g
g![]() ��2
��2![]() dcm��3=
dcm��3=![]() g��cm��3��
g��cm��3��
����ͼ��֪ÿ����������8���������壬�����������е����������϶�ж������ԭ�ӣ���ô�������8��Hԭ�ӣ��Ҵ��ھ����ڲ�����һ������ӵ��4��Tiԭ�ӣ�n(Ti):n(H)=1:2�������γɵ��⻯�ѵĻ�ѧʽΪTiH2��
�ʴ�Ϊ��12�� ![]() ��TiH2��
��TiH2��

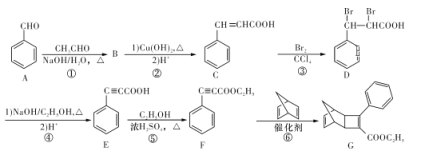
����Ŀ���뵼�幤ҵ��Ҫ�ߴ��裬�ֹ��к���SiO2��Fe2O3��CuO��C�����ʣ��ᴿ��ķ����Ƚ϶࣬�����������(��һ������)�ԭ�������Ȼ����ԭ��Ӧ�ý�Ϊ�㷺��������ʵ����ģ�ҵ����������ԭ���Ļ���ԭ����
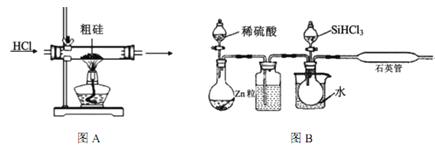
��֪����Si+3HCl ![]() SiHCl3+H2(��Ҫ��Ӧ)
SiHCl3+H2(��Ҫ��Ӧ)
Si+4HCl![]() SiCl4+2H2(��Ҫ��Ӧ)
SiCl4+2H2(��Ҫ��Ӧ)
2NaCl+H2SO4(Ũ)![]() Na2SO4+2HCl��
Na2SO4+2HCl��
�ڵ������Ҫ������IJ����������±���
�۵�(��) | �е�(��) | �ܽ��� | |
SiHCl3 | -127 | 33 | �����ڶ����л��ܼ� |
SiCl4 | -23 | 77 | �����ڶ����л��ܼ� |
(1)�ֹ����ᴿǰ����Ҫ�����ᡢ��Ԥ��������Ŀ����___________________________________��
(2)����װ����ʵ���ҿ������Ʊ�HCl�������_____(����ĸ����)��
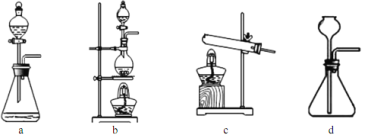
(3)��1100��1200�������£�����ͼB��ʯӢ���п��Ի�ù裬д���÷�Ӧ�ķ���ʽ_______�����ձ���ˮ��������__________________________����װ��ʹ��ʯӢ�ܶ�������ͨ�����ܵ�ԭ����_________��
(4)��������ԭ���� SiHCl3��ͼB���ƹ裬����֮����_______________(����ش�һ�㼴��)��
(5)SiCl4��������ӦҲ�ܵõ��裬����Ӧ�����¶Ȳ�ͬ���ڹ�ҵ����������������л����SiCl4���������������Ӧ������Ϊ��õķ��뷽����______________��
(6)��ҵ����������Ȼ����ԭ���ƹ裬��ԭ�����£�![]() �÷���������������ԭ���Ƚϣ���ȱ���������������ֱ���_________________��
�÷���������������ԭ���Ƚϣ���ȱ���������������ֱ���_________________��
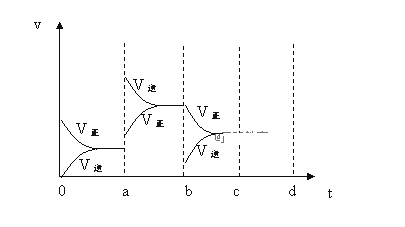
����Ŀ����ӦX(g)![]() 4Y(g)+Z(g)����200���T��ʱ��X �����ʵ���Ũ��(��λ��mol��L-1)��ʱ��� �����й�ʵ�����ݼ��±���
4Y(g)+Z(g)����200���T��ʱ��X �����ʵ���Ũ��(��λ��mol��L-1)��ʱ��� �����й�ʵ�����ݼ��±���
ʱ��/min | 0 | 2 | 4 | 6 | 8 | 10 |
200/�� | 0.80 | 0.55 | 0.35 | 0.20 | 0.15 | 0.15 |
T/�� | 1.00 | 0.65 | 0.35 | 0.18 | 0.18 | 0.18 |
�����йظ÷�Ӧ��������ȷ����
A. ��200��ʱ��4min����Y��ʾ�Ļ�ѧ��Ӧ����Ϊ0.1125mol��L-1��min-1
B. T���£�6minʱ��Ӧ�պôﵽƽ��״̬
C. �����ϱ��� X ��Ũ�ȱ仯����֪Ũ��Խ��Ӧ����Խ��
D. �ӱ��п��Կ���T <200