ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩœψΫΕΙϊ Β≥… λΙΐ≥Χ÷–”…”Ύ¥Δ¥φΒΡΈο÷ ±δΈΣΩ…»ή–‘Χ«¥”Εχ ΙΙϊ ΒΧπΕ»‘ωΦ”ΓΘΡ≥–Υ»Λ–ΓΉιΕ‘¥Υ…ζάμ±δΜ·Ϋχ––ΝΥ≥θ≤ΫΧΫΨΩΘΚ»ΓΈ¥≥… λœψΫΕΙϊ Β»τΗ…Ζ≈‘Ύ “ΥΧθΦΰœ¬»ΟΤδΉ‘»Μ≥… λΘ§ΟΩΧλΕ® ±»Γ10 gΙϊ»β―–ΡΞ≈δ÷Τ≥…100 mL¥ΐ≤β―υ“ΚΤΫΖ÷ΈΣAΓΔBΝΫΖίΘ§Ζ÷±π”ΟΒβ“ΚΚΆλ≥Ν÷ ‘ΦΝΦλ≤βΘ§Φ«¬Φ―’…Ϊ…ν«≥ΒΡ±δΜ·ΓΘ
(1)―υ“ΚA”ΟΒβ“ΚΦλ≤βΒΡΡΩΒΡ «__________________ΘΜ―υ“ΚB”Ολ≥Ν÷ ‘ΦΝΦλ≤βΒΡΡΩΒΡ «___________ΓΘ
(2)λ≥Ν÷ ‘ΦΝ Ι”Ο ±–ηΦΉΓΔ“““ΚΒ»ΝΩΜλΚœΨυ‘»Κσ‘ΌΉΔ»κΘ§≤Δ____________Ιέ≤λ―’…Ϊ±δΜ·ΓΘ
(3)ΗυΨί‘ΛΤΎΫαΙϊ‘ΎΉχ±ξΆΦ÷–Μ≠≥ωΝΫ÷÷―’…ΪΒΡ±δΜ·ΓΘ

ΓΨ¥πΑΗΓΩΦλ≤βΒμΖέΚ§ΝΩΒΡ±δΜ·Φλ≤βΜΙ‘≠Χ«Κ§ΝΩΒΡ±δΜ·Υ°‘ΓΦ”»»
ΓΨΫβΈωΓΩ
λΝΖ’ΤΈ’…ζΈο¥σΖ÷Ή”ΒΡΦλ≤βΩ…“‘ΉΦ»ΖΜΊ¥π¥ΥΧβΓΘ
Θ®1Θ©ΒμΖέ”ωΒβ“Κ±δάΕΘ§ Ι”ΟΒβ“ΚΩ…“‘ΗυΨίάΕ…ΪΒΡ…ν«≥ά¥≈–ΕœΒμΖέΚ§ΝΩΒΡ±δΜ·Θ§λ≥Ν÷ ‘ΦΝ”κΜΙ‘≠–‘Χ«Ω…“‘≤ζ…ζΉ©Κλ…Ϊ≥ΝΒμΘ§Ι Ι”Ολ≥Ν÷ ‘ΦΝΩ…“‘”Οά¥Φλ≤βΜΙ‘≠–‘Χ«Κ§ΝΩΒΡ±δΜ·ΓΘΘ®2Θ©λ≥Ν÷ ‘ΦΝ”κΜΙ‘≠–‘Χ«Ζ¥”Π ±–η“ΣΥ°‘ΓΦ”»»≤≈Ω…“‘≥ωœ÷Ή©Κλ…Ϊ≥ΝΒμΓΘ
Θ®3Θ©‘ΎΙϊ Β≥… λΙΐ≥Χ÷–Θ§Υφ ±Φδ―”≥ΛΘ§ΒμΖέΖ÷Ϋβ≤ΔΉΣΜ·ΈΣΜΙ‘≠Χ«Θ§άΕ…Ϊ÷πΫΞ±δ«≥Θ§Ή©Κλ…Ϊ÷πΫΞΦ”…νΓΘ

 ΤΏ–«ΆΦ ιΩΎΥψΥΌΥψΧλΧλΝΖœΒΝ–¥πΑΗ
ΤΏ–«ΆΦ ιΩΎΥψΥΌΥψΧλΧλΝΖœΒΝ–¥πΑΗΓΨΧβΡΩΓΩΨέΙηΥαΧζ «ΡΩ«ΑΈόΜζΗΏΖ÷Ή”–θΡΐΦΝ―–ΨΩΒΡ»»ΒψΘ§“Μ÷÷”ΟΗ÷Ιή≥ßΒΡΖœΧζ‘ϋΘ®÷ς“Σ≥…Ζ÷Fe3O4Θ§…ΌΝΩCΦΑSiO2Θ©ΈΣ‘≠Νœ÷Τ±ΗΒΡΝς≥Χ»γœ¬ΘΚ
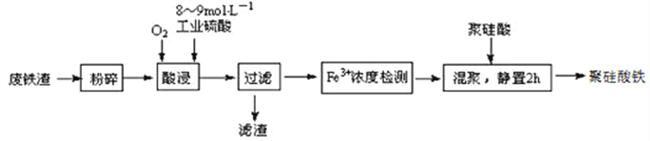
“―÷ΣΘΚ‘Ύ“ΜΕ®Έ¬Ε»œ¬ΥαΫΰ ±Fe3+‘ΎpH=2ΩΣ Φ≥ΝΒμΘ§pH=3.7≥ΝΒμΆξ»Ϊ
Θ®1Θ©ΖœΧζ‘ϋΫχ––ΓΑΖέΥιΓ±ΒΡΡΩΒΡ «___________________________________________________ΓΘ
Θ®2Θ©ΓΑΥαΫΰΓ±–η “ΥΒΡΥα≈®Ε»ΓΔ“ΚΙΧ±»ΓΔΥαΫΰΈ¬Ε»ΓΔ―θΝςΝΩΒ»Θ§Τδ÷–ΥαΫΰΈ¬Ε»Ε‘ΧζΫΰ»Γ¬ ΒΡ”Αœλ»γœ¬±μΥυ ΨΘΚ
Έ¬Ε»Γφ | 40 | 60 | 80 | 100 | 120 |
ΧζΫΰ»Γ¬ | 50 | 62 | 80 | 95 | 85 |
ΔΌ«κ–¥≥ωΥαΫΰΙΐ≥Χ÷–Fe3O4ΖΔ…ζΒΡάκΉ”Ζ¥”ΠΖΫ≥Χ Ϋ__________________________________ΓΘ
ΔΎΝρΥαΥαΫΰ ±”ΠΩΊ÷Τ»ή“ΚΒΡpH____________Θ§Τδ‘≠“ρ «_________________________________ΓΘ
ΔέΒ±ΥαΫΰΈ¬Ε»≥§Ιΐ100Γφ ±Θ§ΧζΫΰ»Γ¬ Ζ¥ΕχΦθ–ΓΘ§Τδ‘≠“ρ «___________________ΓΘ
Θ®3Θ©…œ ωΙΐ¬Υ≤Ϋ÷ηΒΡ¬Υ“ΚΒΡ÷ς“Σ≥…Ζ÷ΈΣ____________Θ®ΧνΜ·―ß ΫΘ©ΓΘ
Θ®4Θ©Fe3+≈®Ε»Ε®ΝΩΦλ‘ρΘ§ «œ»”ΟSnCl2ΫΪFe3+ΜΙ‘≠ΈΣFe2+ΘΜ‘ΎΥα–‘ΧθΦΰœ¬Θ§‘Ό”ΟK2Cr2O7±ξΉΦ»ή“ΚΒΈΕ®Fe2+Θ®Cr2O72-±ΜΜΙ‘≠ΈΣCr3+Θ©Θ§ΗΟΒΈΕ®Ζ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣ____________ΓΘ
ΓΨΧβΡΩΓΩ―œ÷ΊΒΡΈμω≤ΧλΤχΘ§Ηχ»ΥΟ«ΒΡ≥ω––ΦΑ…μΧε‘λ≥…ΝΥΦΪ¥σΒΡΈΘΚΠΘ§―–ΨΩNO2ΓΔSO2ΓΔCOΒ»¥σΤχΈέ»ΨΤχΧεΒΡ–Έ≥…ΦΑ¥ΠάμΨΏ”–÷Ί“Σ“β“εΓΘ
(1)500Γφ ±Θ§‘Ύ¥ΏΜ·ΦΝ¥φ‘ΎΧθΦΰœ¬Θ§Ζ÷±πΫΪ2molSO2ΚΆ1molO2÷Ο”ΎΚψ―Ι»ίΤςΦΉΚΆΚψ»ί»ίΤς““÷–(ΝΫ»ίΤςΤπ Φ»ίΜΐœύΆ§)Θ§≥δΖ÷Ζ¥”ΠΘ§Εΰ’ΏΨυ¥οΒΫΤΫΚβΚσ:
ΔΌΝΫ»ίΤς÷–SO2ΒΡΉΣΜ·¬ ΙΊœΒ «ΦΉ_____““(ΧνΓΑ>Γ±ΓΔΓΑ<Γ±ΜρΓΑ=Γ±)ΓΘ
ΔΎ‘Ύ»ίΤς““÷–Θ§Ζ¥”Π¥οΒΫΤΫΚβΚσΘ§ΗΡ±δœ¬Ν–ΧθΦΰΘ§Ρή ΙSO2ΒΡΉΣΜ·¬ ΧαΗΏΒΡ «____(ΧνΉ÷ΡΗ)ΓΘ
a.Έ¬Ε»ΚΆ»ίΤςΧεΜΐ≤Μ±δΘ§≥δ»κ1.0molHe b.Έ¬Ε»ΚΆ»ίΤςΧεΜΐ≤Μ±δΘ§≥δ»κ1.0molO2
c.‘ΎΤδΥϊΧθΦΰ≤Μ±δ ±Θ§≥δ»κ1molSO3 d.‘ΎΤδΥϊΧθΦΰ≤Μ±δ ±Θ§ΗΡ”ΟΗΏ–ߥΏΜ·ΦΝ
(2)άϊ”ΟΡΤΦν―≠ΜΖΖ®Ω…Ά―≥ΐ―ΧΤχ÷–ΒΡSO2ΓΘ
ΔΌ‘ΎΡΤΦν―≠ΜΖΖ®÷–Θ§Na2SO3»ή“ΚΩ…ΉςΈΣΈϋ ’“ΚΘ§Ω…”…NaOH»ή“ΚΈϋ ’SO2÷ΤΒΟΘ§ΗΟΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ Ϋ «_______________ΓΘ
ΔΎΈϋ ’“ΚΈϋ ’SO2ΒΡΙΐ≥Χ÷–Θ§pHΥφn(SO32-):n(HSO3-)±δΜ·ΙΊœΒ»γœ¬±μ:
n(SO32-):n(HSO3-) | 91:9 | 1:1 | 9:91 |
pH | 8.2 | 7.2 | 6.2 |
”……œ±μ≈–ΕœΘ§NaHSO3»ή“Κœ‘_____–‘(ΧνΓΑΥαΓ±ΓΔΓΑΦνΓ±ΜρΓΑ÷–Γ±)Θ§”ΟΜ·―ßΤΫΚβ‘≠άμΫβ Ά:________ΓΘ
(3)”ΟCH4¥ΏΜ·ΦΝΜΙ‘≠NO2Ω…“‘œϊ≥ΐΒΣ―θΜ·ΒΡΈέ»ΨΘ§άΐ»γ:
CH4(g)+4NO2(g)=4NO(g)+CO2(g)+2H2O(g) ΓςH=-574kJ/mol
CH4(g)+4NO(g)=2N2(g)+CO2(g)+2H2O(g) ΓςH=-1160kJ/mol
»τ”Ο±ξΉΦΉ¥Ωωœ¬4.48CH4ΜΙ‘≠NO2÷ΝN2Θ§’ϊΗωΙΐ≥Χ÷–ΉΣ“ΤΒΡΒγΉ”Ήή ΐΈΣ_____(ΑΔΖϋΦ”Β¬¬ό≥Θ ΐΒΡ÷Β”ΟNA±μ Ψ)Θ§Ζ≈≥ωΒΡ»»ΝΩΈΣ_______kJΓΘ
(4)ΙΛ“Β…œΚœ≥…Α±Υυ–η«βΤχΒΡ÷Τ±ΗΙΐ≥Χ÷–Θ§Τδ÷–ΒΡ“Μ≤ΫΖ¥”ΠΈΣΘΚCO(g)+H2O(g)====CO2(g)+H2(g) ΓςH<0ΓΘ“ΜΕ®ΧθΦΰœ¬Θ§ΫΪCO(g)”κH2O(g)“‘ΧεΜΐ±»ΈΣ1ΘΚ2÷Ο”ΎΟή±’»ίΤς÷–ΖΔ…ζ…œ ωΖ¥”ΠΘ§¥οΒΫΤΫΚβ ±≤βΒΟCO(g)”κH2O(g)ΧεΜΐ±»ΈΣ1:6.‘ρΤΫΚβ≥Θ ΐK=______(ΦΤΥψΫαΙϊ±ΘΝτΝΫΈΜ–Γ ΐ)ΓΘ

