��Ŀ����
����Ŀ����Ļ�����ḻ��ʣ��Ҷ���һ������;��
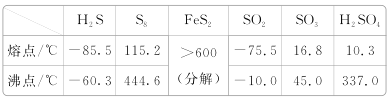
(1)������ҵ����S2Cl2�ķ��ӽṹ��ÿ��ԭ�Ӿ�����8�����ȶ��ṹ��S2Cl2�ĵ���ʽΪ________��S2Cl2��ˮ��������ˮ�ⷴӦ����һ�ֵ���ɫ������������壬�䷴Ӧ�Ļ�ѧ����ʽΪ__________________________________��
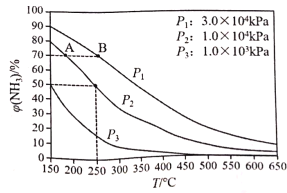
(2)�����������Ĺ��������[(NH4)2S2O8]�������ӻ����������ˮ������������[(NH4)2SO4]Ϊԭ���Ʊ���
��(NH4)2SO4��Һ�����Ե�ԭ����________________________(�����ӷ���ʽ��ʾ)��(NH4)2SO4��Һ�и������ӵ�Ũ���ɴ�С��˳��Ϊ________________________��
�����������Һ��Ũ��Ϊ250 g��L��1�������ʵ���Ũ����________mol��L��1��
��(NH4)2S2O8��Ag���Ĵ��������ܽ�Mn2��������MnO![]() ���䷴Ӧ�����ӷ���ʽΪ__________________________________________________________________��
���䷴Ӧ�����ӷ���ʽΪ__________________________________________________________________��
(3)Na2S2O4�ڿ��������г���������������������������ˮ����������Ӧʱ������ԭ���������������ʵ���֮��Ϊ1��1�������Ϊ________(�ѧʽ)��
���𰸡�![]() 2S2Cl2��2H2O=4HCl����SO2����3S�� NH
2S2Cl2��2H2O=4HCl����SO2����3S�� NH![]() ��H2O NH3��H2O��H�� c(NH4+)��c(SO42-)��c(H��)��c(OH��) 1.89 2Mn2����5S2O82-��8H2O
��H2O NH3��H2O��H�� c(NH4+)��c(SO42-)��c(H��)��c(OH��) 1.89 2Mn2����5S2O82-��8H2O![]() 2MnO4-��10SO42-��16H�� NaHSO4��NaHSO3
2MnO4-��10SO42-��16H�� NaHSO4��NaHSO3
��������
(1)S2Cl2��ÿ��ԭ�Ӿ�����8�����ȶ��ṹ����ṹʽΪCl��S��S��Cl���������֪��S���ɣ�����������ԭ��Ӧ��ˮ�ⷴӦ�Ĺ���֪��HCl��SO2���ɣ�
(2)��(NH4)2SO4Ϊǿ�������Σ���Һ��NH4+ˮ��������ԣ�ˮ��̶�Ϊ������ˮ�⣻
��ת������Ϊ���ʵ������ɣ�
�۸���������ԭ��Ӧ�е�ʧ�����غ��Լ�����غ���ƽ��
(3)���ݵ�ʧ�����غ���ƽ��
(1)S2Cl2��ÿ��ԭ�Ӿ�����8�����ȶ��ṹ����ṹʽΪCl��S��S��Cl�������ʽΪ![]() ���������֪��S���ɣ�����������ԭ��Ӧ��ˮ�ⷴӦ�Ĺ���֪��HCl��SO2���ɣ�������Ӧ�Ļ�ѧ����ʽΪ2S2Cl2��2H2O=4HCl����SO2����3S����
���������֪��S���ɣ�����������ԭ��Ӧ��ˮ�ⷴӦ�Ĺ���֪��HCl��SO2���ɣ�������Ӧ�Ļ�ѧ����ʽΪ2S2Cl2��2H2O=4HCl����SO2����3S����
(2)��(NH4)2SO4��Һ��NH4+ˮ��������ԣ���ˮ�ⷽ��ʽΪNH4+��H2ONH3��H2O��H����(NH4)2SO4ˮ����һ�����Ĺ��̣���c(NH4+)��c(SO42-)��c(H��)��c(OH��)��
��c��![]() mol��L��1��1.89 mol��L��1��
mol��L��1��1.89 mol��L��1��
�۸���������ԭ��Ӧ�е�ʧ�����غ��Լ�����غ�ɵ����ӷ���ʽ2Mn2����5S2O82-��8H2O![]() 2MnO4-��10SO42-��16H����
2MnO4-��10SO42-��16H����
(3) Na2S2O4Ϊ��ԭ����O2Ϊ����������ԭ���������������ʵ���֮��Ϊ1��1ʱ�����ݵ�ʧ�����غ��д����Ӧ�Ļ�ѧ����ʽNa2S2O4��O2��H2O=NaHSO4��NaHSO3������ΪNaHSO4��NaHSO3��

 ����ѵ��ϵ�д�
����ѵ��ϵ�д� ��ĩ�����ϵ�д�
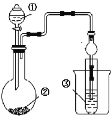
��ĩ�����ϵ�д�����Ŀ������ͼ��ʾװ��(�г���װ��ʡ��)��������ʵ�飬�ܵó���Ӧʵ����۵���
ѡ�� | �� | �� | �� | ʵ����� |
|
A | ϡ���� | Na2S | AgNO3��AgCl����Һ | Ksp(AgCl)>Ksp(Ag2S) | |
B | Ũ���� | ���� | ��ˮ | Ũ���������ˮ�ԡ������� | |
C | ϡ���� | Na2CO3 | CaCl2��Һ | CO2�����Ȼ��Ʒ�Ӧ | |
D | Ũ���� | Na2CO3 | Na2SiO3��Һ | ���ԣ�����>̼��>���� |
A. AB. BC. CD. D