ЬтФПФкШн
МюЪНЬМЫсЭПЩБэЪОЮЊЃКxCuCO3ЁЄyCu(OH)2ЁЄzH2OЃЌВтЖЈМюЪНЬМЫсЭзщГЩЕФЗНЗЈгаЖржж(Cu
ЕФЯрЖддзгжЪСПАД63.5МЦ)ЁЃ
(1)ЯжВЩгУЧтЦјЛЙдЗЈЃЌЧыЛиД№ЯТСаЮЪЬтЃК
вбжЊxCuCO3ЁЄyCu(OH)2ЁЄzH2OгыЧтЦјЗДгІЕФЛЏбЇЗНГЬЪНЮЊxCuCO3ЁЄyCu(OH)2ЁЄzH2OЃЋ(xЃЋy)H2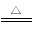 (xЃЋy)CuЃЋxCO2ЃЋ(xЃЋ2yЃЋz)H2O
(xЃЋy)CuЃЋxCO2ЃЋ(xЃЋ2yЃЋz)H2O
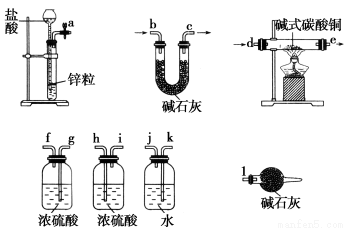
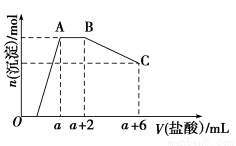
ЂйЪЕбщзАжУгУЯТСаЫљгавЧЦїСЌНгЖјГЩЃЌАДЧтЦјСїЗНЯђЕФСЌНгЫГађЪЧ(ЬюШывЧЦїНгПкзжФИБрКХ)ЃК
(a)Ёњ(ЁЁЁЁ)(ЁЁЁЁ)Ёњ(ЁЁЁЁ)(ЁЁЁЁ)Ёњ(ЁЁЁЁ)(ЁЁЁЁ)Ёњ(ЁЁЁЁ)(ЁЁЁЁ)Ёњ(ЁЁЁЁ)(ЁЁЁЁ)Ёњ(l)ЃЛ
ЂкГЦШЁ23.9 gФГМюЪНЬМЫсЭбљЦЗЃЌГфЗжЗДгІКѓЕУЕН12.7 gВаСєЮяЃЌЩњГЩ4.4 gЖўбѕЛЏЬМКЭ7.2 gЫЎЁЃИУбљЦЗЕФНсОЇЫЎжЪСПЮЊ________gЃЌЛЏбЇЪНЮЊ________ЁЃ
(2)ФГЭЌбЇвдЕЊЦјДњЬцЧтЦјЃЌВЂгУЩЯЪіШЋВПЛђВПЗжвЧЦїРДВтЖЈМюЪНЬМЫсЭЕФзщГЩЃЌФуШЯЮЊЪЧЗёПЩааЃПЧыЫЕУїРэгЩ_________________________________________________________
(1)ЂйkЁЁjЁЁg(Лђh)ЁЁf(Лђi)ЁЁdЁЁeЁЁh(Лђg)ЁЁi(Лђf)ЁЁbЁЁc
Ђк1.8ЁЁCuCO3ЁЄCu(OH)2ЁЄH2O
(2)ПЩааЁЃИљОнЗДгІxCuCO3ЁЄyCu(OH)2ЁЄzH2O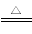 (xЃЋy)CuOЃЋxCO2ЁќЃЋ(yЃЋz)H2OЁќЃЌвРОнМюЪНЬМЫсЭЁЂCuOЁЂCO2КЭH2OЕФжЪСП(ЛђЦфжаШЮвтШ§ИіСП)ЃЌМДПЩМЦЫуГіЦфзщГЩ
(xЃЋy)CuOЃЋxCO2ЁќЃЋ(yЃЋz)H2OЁќЃЌвРОнМюЪНЬМЫсЭЁЂCuOЁЂCO2КЭH2OЕФжЪСП(ЛђЦфжаШЮвтШ§ИіСП)ЃЌМДПЩМЦЫуГіЦфзщГЩ
ЁОНтЮіЁП(1)ЂйгЩЪЕбщФПЕФКЭЪЕбщзАжУжЊЃЌИУЪЕбщвЊВтЖЈЩњГЩЕФЫЎЁЂЖўбѕЛЏЬМЕФСПЃЌЖјДгaПкГіРДЕФЧтЦјжаКЌгаЫЎеєЦјКЭЛгЗЂГіРДЕФТШЛЏЧтЃЌЛсЖдЪЕбщгаИЩШХЃЌЫљвдгІЯШгУЫЎЮќЪеТШЛЏЧтЃЌдйгУХЈСђЫсЮќЪеЫЎеєЦјЃЌдйЭЈШыМюЪНЬМЫсЭжаЗЂЩњЗДгІЃЌЖјВњЩњЕФСНжжЦјЬхгІЯШгУХЈСђЫсЮќЪеЫЎеєЦјЃЌдйгУUаЮЙмжаЕФМюЪЏЛвЮќЪеЖўбѕЛЏЬМЃЌЭЌЪБвЊСЌНгЩЯзАгаМюЪЏЛвЕФЧђаЮИЩдяЙмЃЌЗРжЙПеЦјжаЕФЫЎеєЦјКЭЖўбѕЛЏЬМНјШыUаЮЙмЖјВњЩњЮѓВюЁЃЂкИљОнЫљИјЪ§ОнПЩМЦЫуГіЩњГЩЕФЭЁЂЖўбѕЛЏЬМЁЂЫЎЕФЮяжЪЕФСПЗжБ№ЮЊ0.2 molЁЂ1 molЁЂ0.4 molЃЌЫљвдПЩжЊ(xЃЋy)ЁУxЁУ(xЃЋ2yЃЋz)ЃН2ЁУ1ЁУ4ЃЌЙЪxЁЂyЁЂzЗжБ№ЮЊ1ЁЂ1ЁЂ1ЃЌДгЖјЧѓЕУМюЪНЬМЫсЭбљЦЗЕФЛЏбЇЪНМАНсОЇЫЎЕФжЪСПЁЃ
(2)ШєвдЕЊЦјДњЬцЧтЦјЃЌдђдРэЪЧРћгУМюЪНЬМЫсЭШШЗжНтЕФЛЏбЇЗНГЬЪНЃК
xCuCO3ЁЄyCu(OH)2ЁЄzH2O (xЃЋy)CuOЃЋxCO2ЁќЃЋ(yЃЋz)H2OЁќЃЌвЊЯыЭъГЩЪЕбщФПЕФЃЌжЊЕРМюЪНЬМЫсЭЁЂCuOЁЂCO2КЭH2OЕФжЪСП(ЛђЦфжаШЮвтШ§ИіСП)МДПЩЁЃ
(xЃЋy)CuOЃЋxCO2ЁќЃЋ(yЃЋz)H2OЁќЃЌвЊЯыЭъГЩЪЕбщФПЕФЃЌжЊЕРМюЪНЬМЫсЭЁЂCuOЁЂCO2КЭH2OЕФжЪСП(ЛђЦфжаШЮвтШ§ИіСП)МДПЩЁЃ