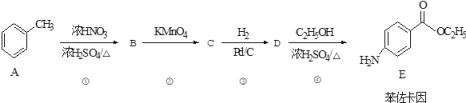
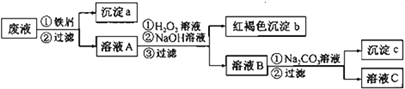
��Ŀ����
����Ŀ�����Т�Na2S���ڽ��ʯ����NH4Cl����Na2SO4���ݸɱ�����Ƭ�������ʣ�������Ҫ��ش�
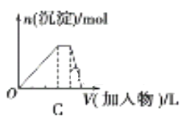
(1)�ۻ�ʱ����Ҫ�ƻ���ѧ������____________���ۻ�ʱ��Ҫ�ƻ����ۼ�����____________���۵���ߵ���____________���۵���͵���_____________(�����)��
(2)�������ӻ��������_______________________��ֻ�����Ӽ���������____________���Է��Ӽ���������ϵ�������____________________(�������)���۵ĵ���ʽ____________________��
(3)�õ���ʽ��ʾ�ٵ��γɹ�����______________________���õ���ʽ��ʾ���γɹ�����_______________________��
���𰸡��ݢ� �� �� �� �٢ۢ� �� �ݢ� 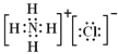
![]()
![]()
��������
��1�����Ӿ����ۻ�ʱ����Ҫ�ƻ���ѧ����ԭ�Ӿ����ۻ�ʱ��Ҫ�ƻ���ѧ����ԭ�Ӿ����۵���ߣ����Ӿ�����۵�һ��ϵͣ�
��2���������Ӽ��Ļ����������ӻ�������ý����ͻ��÷ǽ���Ԫ��֮�����γ����Ӽ������Ӿ����з���֮���Է��Ӽ���������ϣ�
��3�����������Ӿ��壬�����Ӻ�������֮�������Ӽ���ϣ��ⵥ���Ƿ��Ӿ��壬�������Iԭ��֮���Թ��ۼ���ϡ�
��1�����Ӿ����ۻ�ʱ����Ҫ�ƻ���ѧ�����ɱ��͵�Ƭ�����ڷ��Ӿ��壬�ۻ�ʱ����Ҫ�ƻ���ѧ����ԭ�Ӿ����ۻ�ʱ��Ҫ�ƻ���ѧ�������ʯ����ԭ�Ӿ��壬�ۻ�ʱ��Ҫ�ƻ����ۼ���ԭ�Ӿ����۵���ߣ����ʯ����ԭ�Ӿ��壬���۵���ߣ��ɱ��Ƿ��Ӿ����ҳ�����Ϊ������̼���壬�۵���ͣ��ʴ�Ϊ���ݢޣ��ڣ��ڣ��ݣ�
��2���⼸���������������ӻ��������Na2S��NH4Cl��Na2SO4��ֻ�����Ӽ�����Na2S�����ڷ��Ӿ�����Ǹɱ��͵�Ƭ�����Ծ����Է��Ӽ���������ϣ���NH4Cl�ĵ���ʽΪ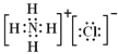 ���ʴ�Ϊ���٢ۢܣ��٣��ݢޣ�
���ʴ�Ϊ���٢ۢܣ��٣��ݢޣ�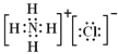 ��
��
��3������Na2S���������Ӿ��壬�����Ӻ�������֮�������Ӽ���ϣ����Ƶ��γɹ��̿ɱ�ʾΪ![]() �����ǵ�Ƭ�����ڷ��Ӿ��壬�ɵ�ԭ��֮���γɹ��ۼ����γɹ���Ϊ
�����ǵ�Ƭ�����ڷ��Ӿ��壬�ɵ�ԭ��֮���γɹ��ۼ����γɹ���Ϊ![]() ��
��