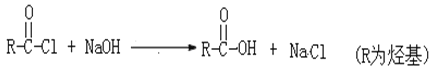
��Ŀ����
����Ŀ��ij���ʳ�������ˮ�еĵ�Ԫ�ض���NH4+��NH3��H2O����ʽ���ڣ��÷�ˮ�Ĵ����������£�

��1��������:��NaOH��Һ�������������ӷ���ʽ��ʾΪ____________������pH��9��������30����ͨ���������ϳ������գ�
��2��������:���������õ������£�NH4+����������Ӧ��������NO3-��������Ӧ�������仯ʾ��ͼ���£�
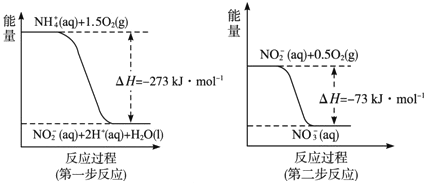
����һ����Ӧ���Ȼ�ѧ����ʽ��____________��
��1molNH4+(aq)ȫ��������NO3-(aq)���Ȼ�ѧ����ʽ��____________��
��3��������:һ�������£����ˮ�м���CH3OH����HNO3��ԭ��N2�����÷�Ӧ����32gCH3OHת��6mol���ӣ���÷�Ӧ�Ļ�ѧ����ʽ��____________��
���𰸡�
��1��NH4++OH����NH3��H2O
��2����NH4+(aq)+3/2O2(g) ��NO2��+2H+( aq)+ H2O(l) ��H=-273kJ/mol
��NH4+(aq)+2O2(g) ��NO3��+2H+( aq)+ H2O(l) ��H=-346 kJ/mol
��3��5CH3OH+6HNO3=3 N2+5CO2+13H2O
��������
�����������1��������ܺ�ǿ�Ӧ��ʵ���ǣ�NH4++OH-�TNH3H2O���ʴ�Ϊ��NH4++OH-�TNH3H2O��
��2������һ�����Ȼ�ѧ����ʽΪNH4+(aq)+1.5O2(g)�TNO2-(aq)+2H+(aq)+H2O(l)����H=-273KJ/mol���ʴ�Ϊ��NH4+(aq)+3/2O2(g) ��NO2��+2H+( aq)+ H2O(l) ��H=-273kJ/mol��
���ڶ������Ȼ�ѧ����ʽΪ��NO2-(aq)+0.5O2(g)�TNO3-(aq)����H=-73KJ/mol��
���ݸ�˹������NH4+(aq)+2O2(g)�T2H+(aq)+H2O(l)+NO3-(aq)����H=-346 kJ/mol��
�ʴ�Ϊ��NH4+(aq)+2O2(g)�T2H+(aq)+H2O(l)+NO3-(aq)����H=-346 kJ/mol��
��3������32g(1mol)CH3OHת��6mol���ӣ���CH3OH��̼ԭ�ӵĻ��ϼ�����6�����Է�Ӧ��̼�Ļ��ϼ�Ϊ+4������ΪCO2�����������غ�ͻ��ϼ�����������ȵã�5CH3OH+6HNO3�T5CO2+3N2+13H2O�� �ʴ�Ϊ��5CH3OH+6HNO3=3 N2+5CO2+13H2O��