ƒøƒ⁄»ð
°æƒø°ø”–ª˙ŒÔG «ƒ≥÷÷“©ŒÔ∫œ≥…µƒ÷–º‰Ã£¨∆‰∫œ≥…¬∑œþ»Áœ¬£∫
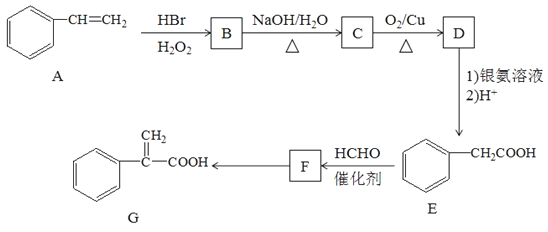
“—÷™£∫¢ŸCH3-CH=CH2+HBr![]() CH3-CH2-CH2Br
CH3-CH2-CH2Br
¢⁄CH3COOH+HCHO![]() HOCH2CH2COOH
HOCH2CH2COOH
«Îªÿ¥œ¬¡–Œ £∫
£®1£©Aµƒ√˚≥∆Œ™___________________________°£
£®2£©F÷–πŸƒÐÕ≈√˚≥∆ «______________________________£ªE◊™ªØFµƒ∑¥”¶¿ý–Õ «_________________°£
£®3£©F◊™ªØŒ™GµƒªØ—ß∑Ω≥Ã Ω£∫_________________________________________°£
£®4£©ªØ∫œŒÔF”–∂ý÷÷Õ¨∑÷“Ïππ㨬˙◊„œ¬¡–Ãıº˛µƒÕ¨∑÷“ÏππÔ–__________÷÷°£
¢Ÿ Ù”⁄∑ºœ„◊ªØ∫œŒÔ«“±Ωª∑…œ”–¡Ω∏ˆ»°¥˙ª˘£ª
¢⁄ƒÐ∑¢…˙ÀÆΩ‚∑¥”¶∫Õ“¯æµ∑¥”¶£ª
¢€”ÎFeCl3»Ð“∫∑¢…˙œ‘…´∑¥”¶°£
∆‰÷–∫À¥≈π≤’Ò«‚∆◊Õº”–6◊È∑«“∑Â√ʪ˝÷Ʊ»Œ™1£∫2£∫2£∫2£∫2£∫1µƒ”–ª˙ŒÔµƒΩ·ππºÚ ΩŒ™__________________£ª
£®5£©≤Œ’’…œ ˆ∫œ≥…¬∑œþ…˺∆“ªÃı”…CH3CHBrCH3∫ÕC2H5OH÷∆±∏CH3CH2COOC2H5µƒ∫œ≥…¬∑œþ£®∆‰À˚ ‘º¡◊‘—°£©______________
°æ¥∞∏°ø±Ω““œ© Ù«ª˘°¢Ù»ª˘ º”≥…∑¥”¶ £∫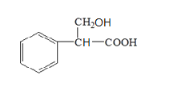
![]()
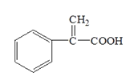 +H2O£ª 6
+H2O£ª 6 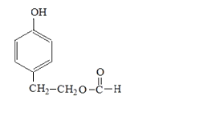 CH3CHBrCH3
CH3CHBrCH3![]() CH3CH=CH2+H2O
CH3CH=CH2+H2O![]() CH3CH2CH2OH
CH3CH2CH2OH![]() CH3CH2CHO
CH3CH2CHO![]() CH3CH2COOH+C2H5OH
CH3CH2COOH+C2H5OH![]() CH3CH2COOC2H5
CH3CH2COOC2H5
°æΩ‚Œˆ°ø
¿ý±»¢Ÿ∑¥”¶ø…÷™£¨BŒ™![]() £¨«‚—ıªØƒ∆ÀƻГ∫º”»»£¨∑¢…˙ÀÆΩ‚∑¥”¶µ√µΩCŒ™
£¨«‚—ıªØƒ∆ÀƻГ∫º”»»£¨∑¢…˙ÀÆΩ‚∑¥”¶µ√µΩCŒ™![]() £¨Cæ≠π˝—ıªØµ√µΩDŒ™
£¨Cæ≠π˝—ıªØµ√µΩDŒ™![]() £¨D±ª—ıªØµ√µΩE°£¿ý±»¢⁄∑¥”¶£¨E”ÎHCHO∑¥”¶µ√µΩFŒ™
£¨D±ª—ıªØµ√µΩE°£¿ý±»¢⁄∑¥”¶£¨E”ÎHCHO∑¥”¶µ√µΩFŒ™ £¨F‘⁄«‚—ıªØƒ∆¥º»Ð“∫º”»»Ãıº˛œ¬∑¢…˙œ˚»•∑¥”¶µ√µΩG£¨æð¥À∑÷Œˆ£ª
£¨F‘⁄«‚—ıªØƒ∆¥º»Ð“∫º”»»Ãıº˛œ¬∑¢…˙œ˚»•∑¥”¶µ√µΩG£¨æð¥À∑÷Œˆ£ª
£®1£©Aµƒ√˚≥∆Œ™±Ω““œ©£ª
£®2£©FŒ™ £¨F÷–πŸƒÐÕ≈√˚≥∆ «Ù«ª˘∫ÕÙ»ª˘£ªE◊™ªØF ±£¨¥Úø™¡À»©ª˘÷–µƒÃº—ıÀ´º¸£¨ Ù”⁄º”≥…∑¥”¶£ª
£¨F÷–πŸƒÐÕ≈√˚≥∆ «Ù«ª˘∫ÕÙ»ª˘£ªE◊™ªØF ±£¨¥Úø™¡À»©ª˘÷–µƒÃº—ıÀ´º¸£¨ Ù”⁄º”≥…∑¥”¶£ª
£®3£©F◊™ªØŒ™GµƒªØ—ß∑Ω≥Ã ΩŒ™£∫
![]()
 +H2O£ª
+H2O£ª
£®4£©ªØ∫œŒÔF”–∂ý÷÷Õ¨∑÷“Ïππ㨢Ÿ Ù”⁄∑ºœ„◊ªØ∫œŒÔ«“±Ωª∑…œ”–¡Ω∏ˆ»°¥˙ª˘£ª¢⁄ƒÐ∑¢…˙ÀÆΩ‚∑¥”¶∫Õ“¯æµ∑¥”¶£ª¢€”ÎFeCl3»Ð“∫∑¢…˙œ‘…´∑¥”¶°£Ω·∫œ»˝∏ˆÃıº˛Õ∆∂œ≥ˆ∏√”–ª˙ŒÔ÷–±Ωª∑…œ”–¡Ω∏ˆ»°¥˙ª˘£¨∆‰÷–“ª∏ˆŒ™Ù«ª˘£¨∫¨”–»©ª˘∫Õı•ª˘£¨∏˘æð∫À¥≈π≤’Ò«‚∆◊Õº”–6◊È∑«“∑Â√ʪ˝÷Ʊ»Œ™1£∫2£∫2£∫2£∫2£∫1£¨Õ∆∂œ≥ˆ¡Ω∏ˆ»°¥˙ª˘¥¶”⁄∂‘ŒªŒª÷√£¨π ¥∞∏Œ™ £ª
£ª
£®5£©”…CH3CHBrCH3∫ÕC2H5OH÷∆±∏CH3CH2COOC2H5ø…œ»»√CH3CHBrCH3∑¢…˙œ˚»•∑¥”¶µ√µΩœ©Ã˛£¨∫ÕÀƺ”≥…µ√µΩÙ«ª˘£¨‘⁄æ≠π˝¡¨–¯—ıªØµ√µΩÙ»ª˘£¨±˚À·”Γ“¥ºı•ªØµ√µΩCH3CH2COOC2H5£¨“Ú¥À£¨¥∞∏Œ™£∫CH3CHBrCH3![]() CH3CH=CH2+H2O
CH3CH=CH2+H2O![]() CH3CH2CH2OH
CH3CH2CH2OH![]() CH3CH2CHO
CH3CH2CHO![]() CH3CH2COOH+C2H5OH
CH3CH2COOH+C2H5OH![]() CH3CH2COOC2H5£ª
CH3CH2COOC2H5£ª

 ≤Ω≤Ω∏þø⁄À„Âø®œµ¡–¥∞∏
≤Ω≤Ω∏þø⁄À„Âø®œµ¡–¥∞∏ µ„涖¬ΩÃ≤ƒ»´ƒÐΩ‚∂¡œµ¡–¥∞∏
µ„涖¬ΩÃ≤ƒ»´ƒÐΩ‚∂¡œµ¡–¥∞∏ –°—ßΩÃ≤ƒÕÍ»´Ω‚∂¡œµ¡–¥∞∏
–°—ßΩÃ≤ƒÕÍ»´Ω‚∂¡œµ¡–¥∞∏°æƒø°øœ¬¡–¿Î◊”∑Ω≥Ã Ωµƒ È–¥º∞∆¿º€æ˘∫œ¿Ìµƒ «( )
—°œÓ | ¿Î◊”∑Ω≥Ã Ω | ∆¿º€ |
A | Ω´2 mol Cl2Õ®»Î∫¨1 mol FeI2µƒ»Ð“∫÷–£∫ 2Fe2£´£´2I£≠£´2Cl2===2Fe3£´£´4Cl£≠£´I2 | ’˝»∑£ªCl2π˝¡ø£¨ø…Ω´Fe2£´°¢I£≠æ˘—ıªØ |
B | Ba(HCO3)2»Ð“∫”Î◊„¡øµƒNaOH»Ð“∫∑¥”¶£∫ Ba2£´£´HCO3-£´OH£≠===BaCO3°˝£´H2O | ’˝»∑£ªÀ· Ω—Œ”κÓ∑¥”¶…˙≥…’˝—Œ∫ÕÀÆ |
C | π˝¡øSO2Õ®»ÎNaClO»Ð“∫÷–£∫ SO2£´H2O£´ClO£≠===HClO£´HSO3- | ’˝»∑£ªÀµ√˜À·–‘£∫H2SO3«ø”⁄HClO |
D | 1 mol/LµƒNaAlO2»Ð“∫∫Õ2.5 mol/LµƒHCl»Ð“∫µ»Ãª˝ªÏ∫œ£∫ 2AlO2-£´5H£´===Al3£´£´Al(OH)3°˝£´H2O | ’˝»∑£ªµ⁄“ª≤Ω∑¥”¶∫Õµ⁄∂˛≤Ω∑¥”¶œ˚∫ƒµƒH£´µƒŒÔ÷ µƒ¡ø÷Ʊ»Œ™2°√3 |
A. A B. B C. C D. D
°æƒø°ø‘⁄80°Ê ±£¨Ω´0.40 molµƒÀƒ—ıªØ∂˛µ™∆¯ÃÂ≥‰»Î2 LµƒπÃ∂®»ðª˝µƒ√б’»ð∆˜÷–£¨∏Ù“ª∂Œ ±º‰∂‘∏√»ð∆˜ƒ⁄µƒŒÔ÷ Ω¯––∑÷Œˆ£¨µ√µΩ»Áœ¬ ˝æð£∫
±º‰/s | 0 | 20 | 40 | 60 | 80 | 100 |
c(N2O4)/mol°§L-1 | 0.20 | a | 0.10 | c | d | e |
c(NO2)/mol°§L-1 | 0.00 | 0.12 | 0.20 | 0.20 | 0.20 | 0.20 |
(1)∏√∑¥”¶µƒªØ—ß∑Ω≥Ã ΩŒ™________________________°£
(2)∏√∑¥”¶‘⁄0~20 sƒ⁄N2O4µƒ∆Ωæ˘∑¥”¶ÀŸ¬ Œ™____________°£
(3)‘⁄80°Ê ±∏√∑¥”¶µƒ∆Ω∫‚≥£ ˝K÷µŒ™______________°£
(4)‘⁄20s ±£¨≈®∂»ÏÿQc_________£®ÃÓ°∞>°±°¢°∞<°±ªÚ°∞=°±£©∆Ω∫‚≥£ ˝K°£