��Ŀ����
��1��ʵ�������Ȼ�粒�����ȡ�����Ļ�ѧ����ʽ��______��
��2����4.48L����״��������ͨ��ˮ�еõ�0.05L��Һ��������Һ�����ʵ���Ũ����______��
��3������100mLAlCl3��MgSO4�Ļ����Һ���ֳ����ȷݣ�
��������һ���м���10mL4mol/L�İ�ˮ��ǡ����ȫ��Ӧ������AlCl3�백ˮ��Ӧ�����ӷ���ʽ��______����������1mol/LNaOH��Һ��10mLʱ���������ټ��٣��������ٵ����ӷ���ʽ��______�����ٵij��������ʵ�����______��
������һ���м���amL1mol/L BaCl2��Һ��ʹSO42-������ȫ��a=______��
��2����4.48L����״��������ͨ��ˮ�еõ�0.05L��Һ��������Һ�����ʵ���Ũ����______��
��3������100mLAlCl3��MgSO4�Ļ����Һ���ֳ����ȷݣ�
��������һ���м���10mL4mol/L�İ�ˮ��ǡ����ȫ��Ӧ������AlCl3�백ˮ��Ӧ�����ӷ���ʽ��______����������1mol/LNaOH��Һ��10mLʱ���������ټ��٣��������ٵ����ӷ���ʽ��______�����ٵij��������ʵ�����______��
������һ���м���amL1mol/L BaCl2��Һ��ʹSO42-������ȫ��a=______��
��1���Ȼ�狀��������Ʒ������ֽⷴӦ�������Ȼ��ơ�������ˮ����Ӧ����ʽΪ��Ca��OH��2+2NH4Cl
CaCl2+2H2O+2NH3����
�ʴ�Ϊ��Ca��OH��2+2NH4Cl
CaCl2+2H2O+2NH3����
��2��4.48L����״�������������ʵ���Ϊn=
=
=0.2mol���õ���0.05L��Һ�����ʵ���Ũ����c=
=
=4mol/L��
�ʴ�Ϊ��4mol/L��
��3����������һ���м���10mL 4mol/L�İ�ˮ��ǡ����ȫ��Ӧ��ӦΪA13++3NH3?H2O=Al��OH��3��+3NH4+��Mg2++2NH3?H2O=Mg��OH��2��+2NH4+����������l mol/L NaOH��Һ��10mLʱ���������ټ��٣��������ٵ����ӷ���ʽ��Al��OH��3+OH-=AlO2-+2H2O������õ������������ʵ���Ϊ0.01mol�����Լ��ٵij���Ϊ�ܽ��������������0.01mol��
�ʴ�Ϊ��A13++3NH3?H2O=Al��OH��3��+3NH4+��Al��OH��3+OH-=AlO2-+2H2O��0.01mol��
�����ݢټ���õ������������ʵ���Ϊ0.01mol������һˮ�ϰ����ʵ���0.03mol�����Գ���þ���ӵ�һˮ�ϰ����ʵ���Ϊ0.01mol��þ�������ʵ���Ϊ0.005mol������������þ�������ʵ���Ϊ0.005mol��
�õ�þ�������ʵ���Ϊ0.005mol��ԭ�����Һ�к���������������ʵ���Ϊ0.005mol������Ba2++SO42-=BaSO4������õ�
L��l mol/L=0.005mol��a=5��
�ʴ�Ϊ��5��
| ||
�ʴ�Ϊ��Ca��OH��2+2NH4Cl
| ||
��2��4.48L����״�������������ʵ���Ϊn=
| V |
| Vm |
| 4.48L |
| 22.4L/mol |
| n |
| V |
| 0.2mol |
| 0.05L |
�ʴ�Ϊ��4mol/L��
��3����������һ���м���10mL 4mol/L�İ�ˮ��ǡ����ȫ��Ӧ��ӦΪA13++3NH3?H2O=Al��OH��3��+3NH4+��Mg2++2NH3?H2O=Mg��OH��2��+2NH4+����������l mol/L NaOH��Һ��10mLʱ���������ټ��٣��������ٵ����ӷ���ʽ��Al��OH��3+OH-=AlO2-+2H2O������õ������������ʵ���Ϊ0.01mol�����Լ��ٵij���Ϊ�ܽ��������������0.01mol��
�ʴ�Ϊ��A13++3NH3?H2O=Al��OH��3��+3NH4+��Al��OH��3+OH-=AlO2-+2H2O��0.01mol��
�����ݢټ���õ������������ʵ���Ϊ0.01mol������һˮ�ϰ����ʵ���0.03mol�����Գ���þ���ӵ�һˮ�ϰ����ʵ���Ϊ0.01mol��þ�������ʵ���Ϊ0.005mol������������þ�������ʵ���Ϊ0.005mol��
�õ�þ�������ʵ���Ϊ0.005mol��ԭ�����Һ�к���������������ʵ���Ϊ0.005mol������Ba2++SO42-=BaSO4������õ�
| a |
| 1000 |
�ʴ�Ϊ��5��

��ϰ��ϵ�д�
 ������ÿ�ʱ�Ż���ҵϵ�д�
������ÿ�ʱ�Ż���ҵϵ�д�
�����Ŀ
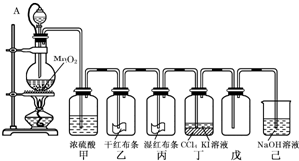
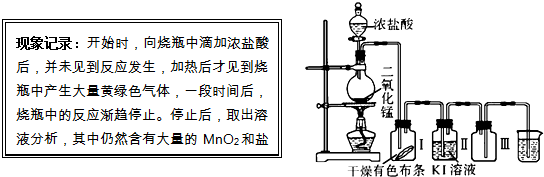
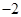 �ۣ���Ԫ����+2��)��ʵ�������û�ͭ��Ϊԭ����ȡ����ͭ������(Fe2O3)���������£�
�ۣ���Ԫ����+2��)��ʵ�������û�ͭ��Ϊԭ����ȡ����ͭ������(Fe2O3)���������£�

 =
=

