��Ŀ����
����Ŀ��ij��ѧ�о���ѧϰС����������װ����ȡ��̽�����������ʡ���Aװ���з�����Ӧ�Ļ�ѧ����ʽΪ2NH4Cl+Ca(OH)2![]() CaCl2+2NH3��+2H2O��
CaCl2+2NH3��+2H2O��
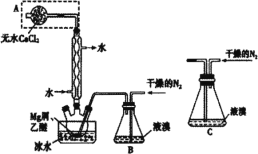
(1)Bװ���еĸ������_________________(������ʯ��������Ũ������)��
(2)C��Dװ������ֽ��ɫ�ᷢ���仯����___________(����C������D��)��
(3)��ʵ�����һ��ʱ���ѹEװ���еĽ�ͷ�ιܣ�����1-2��Ũ���ᣬ�ɹ۲쵽Eװ���е������Dz���_____________(��������������������)��
(4)�ձ�F��ˮ��������________________________��
(5)���Ƶñ�״����11.2 LNH3��������ҪCa(OH)2������Ϊ____________g��
���𰸡���ʯ�� D ���� ���ն����NH3����ֹ��Ⱦ����(���������𰸾�����) 18.5g
��������
��ʵ��Ϊ��ȡ��̽�����������ʣ�����Aװ��ΪNH4Cl��Ca(OH)2��ϼ�����ȡ������װ�ã�BΪ���ﰱ����װ�ã�CΪ����NH3�Ƿ���ʹ����ĺ�ɫʯ����ֽ������װ�ã�DΪ����NH3�Ƿ���ʹʪ��ĺ�ɫʯ����ֽ������װ�ã�EΪ��֤NH3��Ũ����ķ�Ӧװ�ã�FΪβ������װ�ã��ݴ˽����ɷ������
(1)������������֪��BΪ���ﰱ����װ�ã����ڰ����Ǽ������壬һ��ѡ�ü�ʯ�ҽ��и���NH3���ʴ�Ϊ����ʯ�ң�
(2)NH3��ˮ��Ӧ������NH3��H2O���Լ��ԣ���ʹ��ɫʯ�����������NH3����ʹ����ĺ�ɫʯ����ֽ����������ʹʪ��ĺ�ɫʯ����ֽ�������ʴ�Ϊ��D��
(3)Ũ��������NH3��Ӧ����NH4Cl���壬��Ӧ����ʽΪ��NH3+HCl===NH4Cl������Ϊ�������̣��ʴ�Ϊ�����̣�
(4)FΪβ������װ�ã�NH3��������ˮ������H2O���ն����NH3����ֹ��Ⱦ�������ʴ�Ϊ�����ն����NH3����ֹ��Ⱦ����(���������𰸾�����)��
(5)��״����11.2LNH3�����ʵ���Ϊ0.5mol���ɷ�Ӧ����ʽ2NH4Cl+Ca(OH)2![]() CaCl2+2NH3��+2H2O��֪��ÿ����1molNH3������0.5molCa(OH)2������ȡ��״����0.5molNH3��������ҪCa(OH)2�����ʵ���Ϊ0.25mol������Ϊ0.25mol��74g/mol=18.5g���ʴ�Ϊ��18.5��
CaCl2+2NH3��+2H2O��֪��ÿ����1molNH3������0.5molCa(OH)2������ȡ��״����0.5molNH3��������ҪCa(OH)2�����ʵ���Ϊ0.25mol������Ϊ0.25mol��74g/mol=18.5g���ʴ�Ϊ��18.5��

����Ŀ���״���һ�����͵���������ȼ�ϣ���ҵ�Ͽ�ͨ��CO��H2�������Ʊ��״���
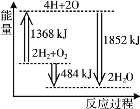
��1����֪����H2(g)��1/2O2(g)![]() H2O(l) ��H1����285.8 kJ/mol��
H2O(l) ��H1����285.8 kJ/mol��
��CO (g)��1/2O2 (g)![]() CO2 (g) ��H2=��283kJ/mol
CO2 (g) ��H2=��283kJ/mol
��CH3OH(g)��3/2O2(g)![]() CO2(g)��2H2O(l) ��H3����764.6 kJ/mol
CO2(g)��2H2O(l) ��H3����764.6 kJ/mol
��ҵ�Ʊ��״��Ŀ��淴Ӧ�Ȼ�ѧ����ʽΪ_______________________________��
��2�����º��������£�������������˵��������Ӧ�Ѵ�ƽ��״̬����__________��
A����λʱ��������n mol CO��ͬʱ����2n mol H2 B����(H2)����2��(CH3OH)��
C��������������ܶȱ��ֲ��� D�������������ѹǿ���ֲ���
��3��ij��ѧ�о���ѧϰС��ģ�ҵ�ϳɼ״��ķ�Ӧ�����ݻ��̶�Ϊ2L���ܱ������ڳ���1 molCO�� 2 molH2��������ʴ�����������Ժ��Բ��ƣ�����250��C��ʼ��Ӧ��CO���ʵ�����ʱ��仯���£�
��Ӧʱ��/min | 0 | 5 | 10 | 15 | 20 | 25 |
n��CO��/mol | 1.00 | 0.79 | 0.63 | 0.54 | 0.50 | 0.50 |
��ӷ�Ӧ��ʼ��20minʱ��������H2��=________�����¶���ƽ�ⳣ��K��_______��
��4�������������250 ����ʼ��Ӧ��CO��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��
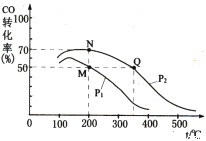
��M��N��Q�����ƽ�ⳣ��KM��KN��KQ�Ĵ�С��ϵΪ___________________________��
����M�㵽N��ı�����������_________��
A�������¶� B������ѹǿ
C�����ø��õĴ��� D��ͨ������CO
��5��25��ʱ��ϡ����Ϊ�������Һ�Ƴɼ״�ȼ�ϵ�أ����ĵ缫����ʽΪ_________________________��
����Ŀ������п�����̵���![]() ��Ҫ�ɷ�ΪZnO����������
��Ҫ�ɷ�ΪZnO����������![]() ��CuO��
��CuO��![]() ��MnO��
��MnO��![]() Ϊԭ�Ͽ���������п����ZnSO4��2H2O��Ħ������Ϊ189g/mol��
Ϊԭ�Ͽ���������п����ZnSO4��2H2O��Ħ������Ϊ189g/mol��

�й��������↑ʼ�����ͳ�����ȫ��pH���±���
�������� |
|
|
|
|
��ʼ������pH |
|
|
|
|
������ȫ��pH |
|
|
|
|
���ʴ��������⣺
(1)����A����Ҫ�ɷ�Ϊ_________������B����Ҫ�ɷ�Ϊ_______________��
(2)���̹����в���![]() ���������ӷ���ʽΪ_______________________��
���������ӷ���ʽΪ_______________________��
(3) �ٳ���![]() ����
����![]() ���ܱ���ȥ
���ܱ���ȥ![]() ʱ����ZnO���Ʒ�ӦҺpH�ķ�ΧΪ______________��
ʱ����ZnO���Ʒ�ӦҺpH�ķ�ΧΪ______________��
����ij��Һ�к���![]() �����ܺ���
�����ܺ���![]() �������ʵ��֤��
�������ʵ��֤��![]() �Ĵ��ڡ�________��
�Ĵ��ڡ�________��
(4)������Ҫ��ø��﴿������п���壬����еIJ�����_______________��
(5)������п������ȷֽ�ɵõ�һ�����ײ��ϡ����ȹ����й�����������¶ȵı仯��ͼ��ʾ��![]() ��Χ�ڣ�������Ӧ�Ļ�ѧ����ʽΪ_________________��
��Χ�ڣ�������Ӧ�Ļ�ѧ����ʽΪ_________________��