��Ŀ����
����Ŀ��A��B��C��D����ѧ��ѧ�еij������ʣ������ʵ�ת����ϵ���£�
![]()
(1)��A�ǻ�ɫ���壬B���γ��������Ҫ����֮һ��
������A��ԭ�ӽṹʾ��ͼ____________��
�� д��D��Ũ��Һ�����е���������________________(���ּ���)��
(2)���˷�Ӧ�����ܽ���ũ�������귢ׯ�����Ļ�ѧԭ����������������ȷ����________��
a.A�Ļ�ѧ���ʽ�Ϊ�ȶ� b.B��C��Ϊ����������
c.�����еķ�Ӧ����������ԭ��Ӧ d.����D�������Ӧ��ȡ����
(3)��A��B��C��D����ɫ��Ӧ��Ϊ��ɫ��CΪ����ɫ���壬A�ڼ��������¿�ֱ��ת��ΪC���� C��D�Ļ�ѧ����ʽ��__________��
(4)��A�ǻ�����Ҵ����̱�ʾ��ҵ�����������ת����ϵ����A��B�ķ�Ӧ����ʽΪ_______________��
���𰸡�![]() ǿ�����ԡ���ˮ�ԡ���ˮ�ԣ����������� ac 2Na2O2+2H2O=4NaOH+O2�� 4NH3+5O2
ǿ�����ԡ���ˮ�ԡ���ˮ�ԣ����������� ac 2Na2O2+2H2O=4NaOH+O2�� 4NH3+5O2![]() 4NO+6H2O
4NO+6H2O
��������
(1)��A�ǻ�ɫ���壬B���γ��������Ҫ�ɷ֣�BΪSO2�����ж�AΪS��DΪ���ᣬCΪSO3��
��AΪ��Ԫ��ԭ�ӣ���Ԫ��Ϊ16��Ԫ�أ�������Ϊ16�����������=������=16��ԭ�Ӻ�����3�����Ӳ㣬����������Ϊ6����ԭ�ӵĽṹʾ��ͼΪ![]() ��
��
��D�����ᣬŨ������е���������Ϊǿ�����ԡ���ˮ�Ժ���ˮ�ԣ�
(2)���˷�Ӧ�����ܽ���ũ�������귢ׯ�����Ļ�ѧԭ������AΪN2��DΪHNO3��BΪNO��CΪNO2����
a��A��N2�������ں�N��N�����û�ѧ���ļ��ܽϴ�����Ҫ�ܸߵ���������˻�ѧ���ʽ�Ϊ�ȶ���a��ȷ��
b��B��C�ֱ���NO��NO2�����߶��Dz����������b����
c�������еķ�Ӧ����Ԫ�صĻ��ϼ۱仯�����Ը÷�Ӧ��������ԭ��Ӧ��c��ȷ��
d��D��HNO3�������������Ӧͨ��������������d����
�ʺ���ѡ����ac��
(3)��A��B��C��D����ɫ��Ӧ��Ϊ��ɫ��CΪ����ɫ���壬A�ڼ��������¿�ֱ��ת��ΪC��AΪNa��DΪNaOH��BΪNa2O��CΪNa2O2����C��D�Ļ�ѧ����ʽ��2Na2O2+2H2O=4NaOH+O2����
(4)A�ǻ�����Ҵ����̱�ʾ��ҵ�����������ת����ϵ����AΪNH3��EΪO2��BΪNO��CΪNO2��DΪ���ᣬA��B�ķ�Ӧ����ʽΪ4NH3+5O2![]() 4NO+6H2O��
4NO+6H2O��

����Ŀ����1���±��ո��е�������ʽΪ_______������_______��ͬ���칹�塣
1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
CH4 | C2H4 | C3H8 | C4H8 | C6H12 | C7H16 | C8H16 |
��2��������(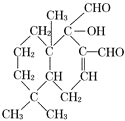 )��һ������ɱ������京�������ŵ�����Ϊ_____________________��
)��һ������ɱ������京�������ŵ�����Ϊ_____________________��
��3��CH2=CH-![]() -C��C-CH3�У������_______��ԭ�ӹ��档
-C��C-CH3�У������_______��ԭ�ӹ��档
��4���ٸ���������д�ṹ��ʽ��2-��-2,4-�Ѷ�ϩ_________________________________
��5���л���![]() ��ϵͳ������___________________________________�������ڴ�����������ȫ�⻯������������ϵͳ������_________________________________
��ϵͳ������___________________________________�������ڴ�����������ȫ�⻯������������ϵͳ������_________________________________
��6��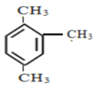 ��ϵͳ������______________________________
��ϵͳ������______________________________
����Ŀ��ij��ѧ��ѧ��ȤС��Ϊ�˵��鵱��ijһ������ˮ����Ⱦ�������ע�������3����ҪˮԴ����ڴ��ɼ�ˮ�����������˷���������������ʵ����Ϣ������һ��ˮԴ����A��B�������ʣ�һ������C��D�������ʣ�һ������E���ʣ�A��B��C��D��EΪ���ֳ�������������±��е������γɣ�
������ | K+��Na+��Cu2+��Al3+ |
������ | SO42-��HCO3-��NO3-��OH- |
Ϊ�˼�������������ֱ��������ʵ�飬�����ǣ�
�ٽ���������ˮ��DΪ��ɫ��Һ��������Ϊ��ɫ��Һ��
�ڽ�E��Һ���뵽C��Һ�г��ְ�ɫ�����������μӣ������ܽ⣻
�۽�����ɫ��Ӧ��ֻ��B��CΪ��ɫ������ɫ�ܲ�������
���ڸ���Һ�м������ᱵ��Һ���ټӹ���ϡ���ᣬA�зų���ɫ���壬C��D�ж��ܲ�����ɫ������
�ݽ�B��D����Һ��ϣ�δ���������������ɡ�
��������ʵ����գ�
��1��д��B�Ļ�ѧʽ��B________��
��2������1 mol A����Һ�뺬1 molE����Һ��Ӧ�����ɣ����õ�һ�ֻ�����û�����Ļ�ѧʽΪ______��
��3����A��Һ�м�����������ʯ��ˮ�������ӷ���ʽΪ________________��
��4��C��������ˮ���������ӷ���ʽ˵���侻ˮԭ��_____________��
��5����������1 mol��C��Һ����μ���Ba(OH)2��Һ�����ɳ����������Ϊ______________g
����Ŀ�����¶ȡ��ݻ���ͬ��3���ܱ������У�����ͬ��ʽͶ�뷴Ӧ����ֺ��¡����ݣ���÷�Ӧ�ﵽƽ��ʱ���й���������[��֪N2(g)��3H2(g)![]() 2NH3(g)����H����92.4 kJ��mol��1]��
2NH3(g)����H����92.4 kJ��mol��1]��
���� | �� | �� | �� |
��Ӧ��Ͷ���� | 1 mol N2��3 mol H2 | 2 mol NH3 | 4 mol NH3 |
NH3��Ũ��(mol��L��1) | c1 | c2 | c3 |
��Ӧ�������仯 | �ų�a kJ | ����b kJ | ����c kJ |
��ϵѹǿ(Pa) | p1 | p2 | p3 |
��Ӧ��ת���� | ��1 | ��2 | ��3 |
����˵����ȷ����
A. 2c1��c3 B. a��b��92.4 C. 2p2��p3 D. ��1����3��1