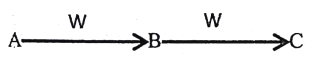��Ŀ����
����Ŀ�������£���100 mL 0.01 mol��L��1HA��Һ����μ���0.02 mol��L��1MOH��Һ��ͼ����ʾ���߱�ʾ�����Һ��pH�仯���(��Һ����仯���Բ���)������˵���У�����ȷ����

A. HAΪһԪǿ��
B. MOHΪһԪ����
C. N��ˮ�ĵ���̶�С��K��ˮ�ĵ���̶�
D. ��K���Ӧ��Һ��pH��10������c(MOH)��c(OH��)��c(H��)��0.005 mol��L��1
���𰸡�C
�������������������ͼ�����֪���������Ϊ51mLʱ��Һ�����ԣ�˵�����߲�����ǿ����ʣ�����Ϊǿ��������50mLʱǡ����ȫ��Ӧ����ʱΪǿ�������Σ���ҺӦ�ʼ��ԣ���ͼ����������ӦΪǿ�ᣬ��Ϊ���A��B��ȷ��C��N��ʱ����Һ�����ԣ�������Ũ�ȵ�������������Ũ��=1��10-7mol/L����K��ʱ�����������ˮ�ĵ��룬����N��ˮ�ĵ���̶ȴ���K��ˮ�ĵ���̶ȣ�����D����K��ʱ���������Ϊ100mL����ʱ��Һ��MOH��MA��Ũ�ȵĻ��Һ��Ũ�ȶ�Ϊ0.005mol/L,���������غ��c(MOH)+c(M+)="0.01" mol/L,���ݵ���غ��c(M+)+c(H+)=c(A-)+c(OH-),��ʽ��ϵ�
c(MOH)+ c(OH-)- c(H+)="0.01-" c(A-)=0.01-0.005=0.005mol/L����ȷ����ѡC��

����Ŀ��25��ʱ��5�����ε��ܶȻ�������Ksp���ֱ��ǣ�
AgCl | Ag2SO4 | Ag2S | AgBr | AgI |
1.8��10-10 | 1.4��10-5 | 6.3��10-50 | 7.7��10-13 | 8.51��10-16 |
����˵����ȷ����
A. �Ȼ������廯���͵⻯�����ܽ����������
B. ���������ܽ���ˮ�������м����������ƹ��壬���ܵõ���ɫ����
C. ��5mL1.5��10-5molL-1��NaCl��Һ�У�����1�Σ�1mLԼ20�Σ�1.0��10-3molL-1��AgNO3��Һ�����ܹ۲쵽��ɫ����
D. ���ձ��з���6.24 g���������壬��200 g ˮ��������ܽ�����ñ�����Һ�����Ϊ200 mL����Һ��Ag + �����ʵ���Ũ��Ϊ0.2molL-1��