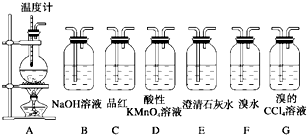
��Ŀ����
ʵ������Ũ������Ҵ�����ϩʱ�����ῴ����ƿ��Һ���ڣ������Ƶõ���ϩ�л���CO2��SO2������
��1��д��ʵ������ȡ��ϩ�Ļ�ѧ����ʽ__________________________________
��2��д������������������Ļ�ѧ����ʽ____________________________��
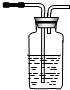
��3�������ͼ��ѡ������Ҫ������(���ظ�ѡ��)���һ���ܽ���������Ӧ����������û�������к�����ϩ��SO2��CO2��H2O(g)��װ��(�����õIJ����ܡ��ܵ���ȥ)����ѡ�õ��Լ���Ũ���ᡢ��ˮ�Ҵ�����ˮ����ͭ������KMnO4��Һ��FeCl3��Һ����ˮ��Ʒ����Һ������ʯ��ˮ��NaOH��Һ��Ũ���ᡣ����������˳����ϵ��½��²���������
��1��д��ʵ������ȡ��ϩ�Ļ�ѧ����ʽ__________________________________
��2��д������������������Ļ�ѧ����ʽ____________________________��
��3�������ͼ��ѡ������Ҫ������(���ظ�ѡ��)���һ���ܽ���������Ӧ����������û�������к�����ϩ��SO2��CO2��H2O(g)��װ��(�����õIJ����ܡ��ܵ���ȥ)����ѡ�õ��Լ���Ũ���ᡢ��ˮ�Ҵ�����ˮ����ͭ������KMnO4��Һ��FeCl3��Һ����ˮ��Ʒ����Һ������ʯ��ˮ��NaOH��Һ��Ũ���ᡣ����������˳����ϵ��½��²���������
��4����˵����������м�����ϩ����CO2��ʵ�������ǣ� ___________________________________________________________________��
��1��CH3CH2OH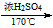 CH2��CH2��+H2O
CH2��CH2��+H2O
��2��C+2H2SO4(Ũ) CO2��+2SO2��+2H2O��C2H5OH+6H2SO4(Ũ)
CO2��+2SO2��+2H2O��C2H5OH+6H2SO4(Ũ) 2CO2����6SO2����9H2O
2CO2����6SO2����9H2O
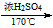 CH2��CH2��+H2O
CH2��CH2��+H2O��2��C+2H2SO4(Ũ)
 CO2��+2SO2��+2H2O��C2H5OH+6H2SO4(Ũ)
CO2��+2SO2��+2H2O��C2H5OH+6H2SO4(Ũ) 2CO2����6SO2����9H2O
2CO2����6SO2����9H2O��3��

��4���ڶ���(�����)Ʒ����Һ����ɫ����ˮ��ɫ������ʯ��ˮ�����

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
ʵ������Ũ������Ҵ���Ӧ�Ʊ���ϩ����ӦΪ��CH3��CH2OH
CH2=CH2��+H2O
���¶ȹ������ʱ��������Ƶõ���ϩ������������CO2��SO2��H2O�����壩����ش��������⣺
I��������ȥ��ϩ�е�CO2��SO2��
II��д����ϩͨ����ˮ�з�Ӧ�Ļ�ѧ����ʽ �÷�Ӧ�ķ�Ӧ����Ϊ
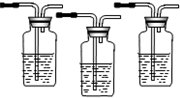
III��������ͼ��ʾ��װ�����һ��ʵ�飬��֤�Ƶõ�������ȷʵ����CO2��SO2��H2O��g����
��1���������ķ���װ�õ�����˳���ǣ� ��
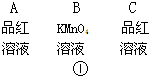
��2��ʵ��ʱ���۲쵽������Aƿ����Һ��ɫ��Bƿ����Һ��ɫ��dz��Cƿ����Һ����ɫ����Aƿ�������� ��Bƿ�������� ��Cƿ�������� ��
��3��װ�â������ӵ��Լ������� ����������֤�������� ��
��4��װ�â�����ʢ��Һ�������� ��������������֤�������� ��
| Ũ���� |
| 170�� |
���¶ȹ������ʱ��������Ƶõ���ϩ������������CO2��SO2��H2O�����壩����ش��������⣺
I��������ȥ��ϩ�е�CO2��SO2��
II��д����ϩͨ����ˮ�з�Ӧ�Ļ�ѧ����ʽ
III��������ͼ��ʾ��װ�����һ��ʵ�飬��֤�Ƶõ�������ȷʵ����CO2��SO2��H2O��g����
��1���������ķ���װ�õ�����˳���ǣ�
  |
�� |
�� |
 �� �� |
��3��װ�â������ӵ��Լ�������
��4��װ�â�����ʢ��Һ��������
ʵ���ҿ����Ҵ�����ȡ��ϩ����ҵ�Ͽ����Ҵ�����ȡ��ȩ��ʵ������Ũ������Ҵ�����ϩʱ�����ῴ����ƿ��Һ���ڣ������Ƶõ���ϩ�л���CO2��SO2�����ʣ����ͼ1��ѡ������Ҫ�����������ظ�ѡ�ã����һ���ܽ���������Ӧ����������û�������к�����ϩ��SO2��CO2��H2O��g����װ�ã������õIJ����ܡ��ܵ���ȥ������ѡ�õ��Լ���Ũ���ᡢ��ˮ�Ҵ�����ˮ����ͭ������KMnO4��Һ��FeCl3��Һ����ˮ��Ʒ����Һ������ʯ��ˮ��NaOH��Һ��Ũ���ᣮ����������˳����ϵ��½��±�����������

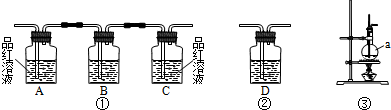
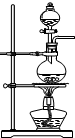
����ͼ2װ�ý����Ҵ��Ĵ�����ʵ�鲢��ȡ��ȩ��ͼ������̨��װ������ȥ���ֺ��߱�ʾ�齺�ܣ�������д���пհף�
��1����װ�ó�������70��80���ˮԡ�У�Ŀ���� ��
��2�����з����ķ�Ӧ�Ļ�ѧ����ʽΪ ��
��3���������ٶȹ������ ��
��4�����Թܶ�����ˮ���ղ����Ҫ�ڵ����ҡ���֮�������װ�ã������ӷ����ǣ�����װ���е��ܴ��ţ����ҽ� �� �ӱ��������ﲻ��ˮ���ն���ֱ����ȴ��Ӧ���Թܶ����� �У������Ҵ�������������Լ��� ���仯ѧ����ʽΪ ��

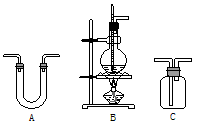
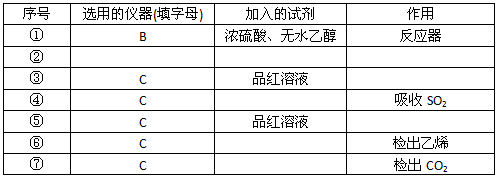
| ��� | ѡ�õ�����������ĸ�� | ������Լ� | ���� |
| �� | B | Ũ���ᡢ��ˮ�Ҵ� | ��Ӧ�� |
| �� | ��ˮ����ͭ | ���ˮ���� | |
| �� | C | Ʒ����Һ | |
| �� | C | ����SO2 | |
| �� | C | Ʒ����Һ | |
| �� | C | �����ϩ | |
| �� | C | ����ʯ��ˮ | ���CO2 |
��1����װ�ó�������70��80���ˮԡ�У�Ŀ����
��2�����з����ķ�Ӧ�Ļ�ѧ����ʽΪ
��3���������ٶȹ������
��4�����Թܶ�����ˮ���ղ����Ҫ�ڵ����ҡ���֮�������װ�ã������ӷ����ǣ�����װ���е��ܴ��ţ����ҽ�