��Ŀ����
�������ƣ�NaClO2����һ����Ҫ�ĺ�������������Ҫ����ˮ�������Լ�ɰ�ǡ���֬��Ư����ɱ���������ǹ������ⷨ�����������ƵĹ�������ͼ��
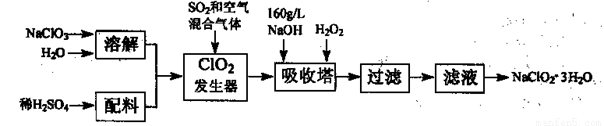
��֪����NaClO2���ܽ�����¶����߶������ʵ������¿ɽᾧ����NaClO2��3H2O��
�ڴ�ClO2�ֽⱬը��һ����ϡ����������ϡ�͵�10%���°�ȫ��
��1���������й�����������ÿ����� ��
��2���������ڷ�����Ӧ�Ļ�ѧ����ʽΪ ��
�������ڵ��¶Ȳ��˹��ߣ�����ᷢ����Ӧ���䷽��ʽΪ�� ��
��3���ڼ�����Һ��NaClO2�Ƚ��ȶ���������������Ӧά��NaOH�Թ������ж�NaOH�Ƿ�����ļ�ʵ�鷽����__ __��
��4��ClO2���������ܽ���Ʒ�ˮ�е�CN������Ϊ�����ʣ���������ԭΪCl����������CNһ��ͬ���ĵ�Ʒ�ˮ�������������ʵ�����ClO2�� ����
��1��ϡ��C1O2�Է�ֹ��ը��3�֣�
��2��2NaOH+2C1O2+H2O2=2NaC1O2+2H2O+O2����3�֣� 2H2O2
2H2O+O2����3�֣�
��3�������ⶨ����������Һ��pH��3�֣�
��4��2.5��3�֣�
��������
�����������1����Ϣָ����ClO2�ֽⱬը��һ����ϡ����������ϡ�͵�10%���°�ȫ�����Թ��������������ϡ��C1O2�Է�ֹ��ը����2��˫��ˮ���ȶ����ȷֽ⣬�������������¶Ȳ��˹��ߣ���3�������ⶨ����������Һ��pH�����ݵ����غ���㣬1molC12�õ�������Ϊ2mol��1molC1O2��õ�5mol���ӣ��ʴ�����CNһ��ͬ���ĵ�Ʒ�ˮ�������������ʵ�����ClO2��2.5����
���㣺���黯�������в���Ŀ�ġ�ԭ�������鼰������й����⡣

 ��У����ϵ�д�
��У����ϵ�д���10�֣��������ȼҵ����Ҫ��Ʒ֮һ������һ�ֳ��õ���������������ԭ������ˮ��Ӧ�����˴����
Cl2 + H2O  HCl + HClO K=4.5��10-4
HCl + HClO K=4.5��10-4
�������ǿ��������ɱ��ˮ�еIJ�������ֱ���ô�����Ϊ����ˮ��������Ϊ�������ֽ⣬�Ҷ��Խϴ����ǣ������������˲����㣬�Ҿ���һ����Σ���ԣ�Ŀǰ��������������Խ��������Ʒ���������ش�
��1���ȼҵ���������Ļ�ѧ����ʽΪ ��
��2��84����Һ(��Ҫ�ɷ�ΪNaClO)��������Ⱦ������˷�����ŵ㣬���������ռ���Һ��Ӧ�Ʊ�84����Һ�����ӷ���ʽΪ ��
��3������������һ�ָ�Ч�����ס���ȫ��ɱ�������ʼ����ҹ���ѧ���з��������������������ƣ�NaClO2�������Ʊ��������ȵķ������仯ѧ����ʽΪ ��
��4��һλͬѧ�����һ����Ũ�����KMnO4������ȡ�����������Ƚ�������ⵥ�ʵ���
����ǿ������װ�ã���ͼ����
��������Һ������Cl2���� ������ĸ��ţ���
| A������ʳ��ˮ | B������Na2SO3��Һ |
| C������NaOH��Һ | D��Ũ���� |
��10�֣��������ȼҵ����Ҫ��Ʒ֮һ������һ�ֳ��õ���������������ԭ������ˮ��Ӧ�����˴����
Cl2 + H2O
 HCl
+ HClO K=4.5��10-4
HCl
+ HClO K=4.5��10-4
�������ǿ��������ɱ��ˮ�еIJ�������ֱ���ô�����Ϊ����ˮ��������Ϊ�������ֽ⣬�Ҷ��Խϴ����ǣ������������˲����㣬�Ҿ���һ����Σ���ԣ�Ŀǰ��������������Խ��������Ʒ���������ش�
��1���ȼҵ���������Ļ�ѧ����ʽΪ ��
��2��84����Һ(��Ҫ�ɷ�ΪNaClO)��������Ⱦ������˷�����ŵ㣬���������ռ���Һ��Ӧ�Ʊ�84����Һ�����ӷ���ʽΪ ��
��3������������һ�ָ�Ч�����ס���ȫ��ɱ�������ʼ����ҹ���ѧ���з��������������������ƣ�NaClO2�������Ʊ��������ȵķ������仯ѧ����ʽΪ ��
��4��һλͬѧ�����һ����Ũ�����KMnO4������ȡ�����������Ƚ�������ⵥ�ʵ���
����ǿ������װ�ã���ͼ����
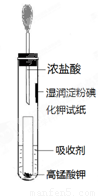
��������Һ������Cl2���� ������ĸ��ţ���
A. ����ʳ��ˮ B. ����Na2SO3��Һ
C. ����NaOH��Һ D. Ũ����
����˵��Cl2��������ǿ��I2��ʵ�������� ��
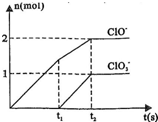 ��2013?����ģ�⣩��֪Ca��OH��2��Cl2��Ӧ�������������¶��йأ���һ������ʯ������ͨ��һ����������������ǡ����ȫ��Ӧ�������ķ�Ӧ��Ϊ���ȷ�Ӧ�����������к���Cl-��ClO-��
��2013?����ģ�⣩��֪Ca��OH��2��Cl2��Ӧ�������������¶��йأ���һ������ʯ������ͨ��һ����������������ǡ����ȫ��Ӧ�������ķ�Ӧ��Ϊ���ȷ�Ӧ�����������к���Cl-��ClO-��