��Ŀ����
����Ŀ����ˮ�DZ������Դ���⣬Ŀǰ�ȼҵ����ˮ��þ����ˮ����Ϊ�����ṩ�˴�����ҵԭ�ϡ���ͼ�Ǻ�ˮ�ۺ����õIJ�������ͼ����ͼ�ش����⣺
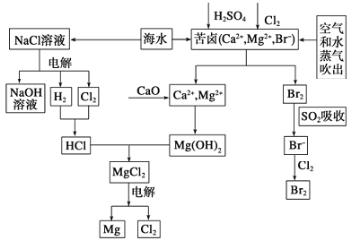
��1���ɺ�ˮɹ�ƵĴ����к���Ca2+��Mg2+��SO42�������ӣ�Ϊ��ȥ��Щ���ӣ������Լ������Ⱥ�˳��Ϊ(д��ѧʽ) ______________________��
��2����Ŀǰ���Ƚ��ĵ���Ƽ�����ӽ���Ĥ��ⷨ�����������ӽ���Ĥ�ѵ��۸��������Һ������ң���������___________��д2��������ⱥ��ʳ��ˮ�Ļ�ѧ��Ӧ����ʽΪ______________��
����ȡMgCl2�Ĺ������漰��Ӧ��MgCl2��6H2O![]() MgCl2+6H2O���÷�ӦҪ��HCl�����н��У�ԭ����_______________��
MgCl2+6H2O���÷�ӦҪ��HCl�����н��У�ԭ����_______________��
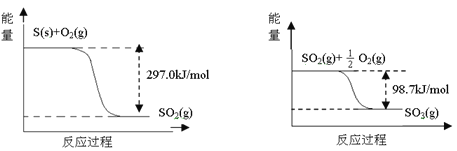
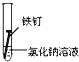
��3����±��ͨ��Cl2�û���Br2����������SO2������д����SO2�������������ӷ���ʽ_________���ɴ��ж�Cl2��Br2��SO2����������ǿ������˳��Ϊ__________________��
��4��Ҳ�й������ڴ���Br2����̼������Һ���գ��γ��廯�ƺ������ƣ�ͬʱ��CO2�ų����÷�Ӧ�����ӷ���ʽ��_____________���������H2SO4�����õ�Br2��֮�����CCl4����Br2����ȡ��������_____________�����õ�����Br2��
���𰸡���1��BaCl2��NaOH��Na2CO3��HCl��
��2������ֹH2��Cl2������Ӧ����������ը����ֹCl2�����ɵ�NaOH��Һ��Ӧ��ʹ�ռ��Ʒ������
2NaCl+2H2O![]() Cl2��+H2��+2NaOH���ڷ�ֹMgCl2ˮ�⣻
Cl2��+H2��+2NaOH���ڷ�ֹMgCl2ˮ�⣻
��3��Br2+SO2+2H2O=2Br-+4H++SO42-��Cl2��Br2��SO2
��4��3Br2+3CO32-�T5Br-+BrO-3+3CO2����������
��������
�����������1��Ca2����Na2CO3��ȥ��Mg2����NaOH��ȥ��SO42����BaCl2��ȥ������BaCl2��Һ��Na2CO3��ȥ�����BaCl2�������Na2CO3����ߣ���������Լ���˳����BaCl2��Na2CO3��NaOH��HCl��NaOH��BaCl2��Na2CO3��HCl��BaCl2��NaOH��Na2CO3��HCl����2���ٵ�ⱥ��ʳ��ˮ��2 NaCl��2H2O![]() 2NaOH��H2����Cl2����Ϊ��ֹH2��Cl2������Ӧ����������ը����ֹCl2�����ɵ�NaOH��Һ��Ӧ��ʹ�ռ��Ʒ��������MgCl2��2H2O
2NaOH��H2����Cl2����Ϊ��ֹH2��Cl2������Ӧ����������ը����ֹCl2�����ɵ�NaOH��Һ��Ӧ��ʹ�ռ��Ʒ��������MgCl2��2H2O![]() Mg(OH)2��2HCl�����ȴٽ�ˮ�⣬�����HCl�ķ�Χ�м��ȣ���ֹMgCl2ˮ�⣻(3)����SO2�Ļ�ԭ�ԣ������壬�������ӷ�Ӧ��Br2+SO2+2H2O=2Br��+4H�� +SO42��������������ԭ��Ӧ��ǿ�����ɣ�Cl2>Br2>SO2��(4)���ݷ�Ӧ��Ϣ���ó���Ӧ����ʽ��3Br2+3CO32-�T5Br-+BrO-3+3CO2�������÷е㲻ͬ����������ķ������з��롣
Mg(OH)2��2HCl�����ȴٽ�ˮ�⣬�����HCl�ķ�Χ�м��ȣ���ֹMgCl2ˮ�⣻(3)����SO2�Ļ�ԭ�ԣ������壬�������ӷ�Ӧ��Br2+SO2+2H2O=2Br��+4H�� +SO42��������������ԭ��Ӧ��ǿ�����ɣ�Cl2>Br2>SO2��(4)���ݷ�Ӧ��Ϣ���ó���Ӧ����ʽ��3Br2+3CO32-�T5Br-+BrO-3+3CO2�������÷е㲻ͬ����������ķ������з��롣

����Ŀ��ȫ����ÿ���������ʴ��ɴ�������ʧ��ijѧ����̽��������ˮ���Ȼ�����Һ�ʹ�����Һ��������������ʴ�Ŀ�������������ʵ�飮
ʵ����� | �� | �� | �� |
ʵ�� ���� |
|
|
|
��ش�
����һ�ܵĹ۲�����У�������ʵ�����Ϊ ���Թ���������ʴ�ٶ���������
�����з�ֹ������ʴ�Ĵ�ʩ���������� ������ĸ����
A�������г��ĸ�Ȧ�������
B���ı�����ڲ��ṹ�Ƴɲ���֣�
C���ڵ��¸����ܵ�������ͭ��
�۳����˵�����δ��ʱϴ������Һ�к�NaCl�����ڶ�������ʴ���ֺ��ɫ��ߣ��Իش������ĸ�ʴ��Ҫ���� ��ʴ��ɵģ�
����Ŀ����������ͼ�У�������Ϊij��Һ�м���ij���ʵ�����������Ϊ���ɳ�����������A��F��ѡ����ϱ��и���Ҫ������������У�
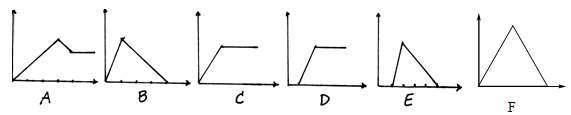
�� Һ | ��������� | ����� |
������ʯ��ˮ | CO2 | |
��AlCl3��Һ | ����NH3 | |
��������NaOH��NaAlO2 | ����CO2 | |
��������NaOH��NaAlO2 | ��μ�ϡ���� | |
��MgCl2��AlCl3���Һ | ��μ�NaOH������ | |
��NaOH��Һ | ��μ�AlCl3������ |