��Ŀ����
����̼������ͭ��һ���¶��·�Ӧʱ������ͭ�ɱ���ԭΪCu2O��Cu���ֽ�2.00g C��16.0g CuO�Ļ���������������һ��ʱ���,�����ɵ�����ͨ�������ijΜ[ʯ��ˮ�����ռ���1.12 L����(��״����,���ɳ���������Ϊ5.00 g������˵���������
| A����Ӧ��Ĺ��������л�����̼ |
| B����Ӧ��Ĺ���������Cu������Ϊ12.8 g |
| C����Ӧ��Ĺ������������FΪ14.4 g |
| D����Ӧ��Ĺ�������������������ʵ���Ϊ0.05mol |
B
�������������A��1.12L������CO�����ʵ�����1.12L��22.4L/mol��0.05mol��5.00g������̼��ƣ����ʵ�����5.00g��100g/mol��0.05mol����CO��̼�����̼Ԫ�ص�����֮���ǣ�0.05mol��0.05mol����12g/mol��1.2g��2.00g������̼����������Ӧ��Ĺ��������л�����̼��A��ȷ��B���跴Ӧ��Cu2O��Cu�����ʵ����ֱ���x��y������ݵ��ӵ�ʧ�غ��֪2x��2y��2��0.05mol��4��0.05mol��0.3mol������ͭԭ���غ��֪2x��y��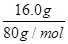 ��0.2mol�����x��0.05mol��y��0.1mol������ ��Ӧ��Ĺ���������Cu������Ϊ0.1mol��64g/mol��6.4g��B����ȷ��C����Ӧ��Ĺ�������������Ϊ2.00g��1.20g��6.4g��0.05mol��144g/mol��14.4 g��C��ȷ��D���������Ϸ�����֪��������ͭ�����ʵ�����0.05mol��D��ȷ����ѡB��
��0.2mol�����x��0.05mol��y��0.1mol������ ��Ӧ��Ĺ���������Cu������Ϊ0.1mol��64g/mol��6.4g��B����ȷ��C����Ӧ��Ĺ�������������Ϊ2.00g��1.20g��6.4g��0.05mol��144g/mol��14.4 g��C��ȷ��D���������Ϸ�����֪��������ͭ�����ʵ�����0.05mol��D��ȷ����ѡB��
���㣺����̼������ͭ��Ӧ���йؼ���

��500mLNH4HCO3��Na2CO3�Ļ����Һ�ֳ���ȷݣ�ȡһ�ݼ��뺬amol�������Ƶ���Һǡ�÷�Ӧ��ȫ����ȡһ�ݼ��뺬b mol HCl������ǡ�÷�Ӧ��ȫ����û����Һ��c(Na+)Ϊ�� ��
A����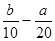 ��mol/L ��mol/L | B��(2b��a)mol/L | C��(l0b - 5a) mol/L | D����5bһ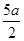 ��mol/L ��mol/L |
��t ��ʱ����a g NH3��ȫ����ˮ���õ�V mL��Һ���������Һ���ܶ�Ϊ�� g��mL��1����������Ϊw�����к���NH4+�����ʵ�����b mol������������ȷ���ǣ� ��
A�����ʵ���������w��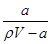 ��100% ��100% |
B�����ʵ����ʵ���Ũ��c�� mol��L��1 mol��L��1 |
C����Һ��c(OH��)��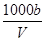 mol��L��1��c(H��) mol��L��1��c(H��) |
| D����������Һ�м���V mLˮ��������Һ��������������0.5w |
��NAΪ�����ӵ�������ֵ������˵������ȷ����
| A����״���£�2.24L��Ȳ�к��Ҽ���ĿΪ0.1 NA |
| B�����³�ѹ�£�1.6gO2������ԭ����ĿΪ0.1NA |
| C��5.6gFe������ϡ���ᷴӦ��ת�Ƶ�����ĿΪ0.2NA |
| D��1L0.1mol��L��1Na2CO3��Һ�к���������������ĿΪ0.3NA |
����˵����ȷ����
| A����ͬ���ʵ�����O2��O3�������ͬ |
| B�����ӻ�����һ���������Ӽ������ܺ��й��ۼ� |
| C�������������ǿ������������ԣ�����֪�����ԣ�Cl2>S |
| D�������£��������pH��Ϊ3�Ĵ��������ֱ�����ȫ��ͬ��þ����Ӧ����������������ʿ� |
��һϡ�����ϡ����Ļ���ᣬ����H2SO4��HNO3�����ʵ���Ũ�ȷֱ���4mol��L��2mol��L��ȡ10mL�˻���ᣬ�����м�����������ۣ�����Ӧ�����ɲ�����״������������Ϊ���跴Ӧ��HNO3����ԭ��NO���� ��
| A��0.448L | B��0.672L | C��0.896L | D��0.224L |
��NAΪ����٤��������ֵ������������ȷ����( )
| A��20mL 10 mol/LŨ�����Ũ����������ͭ���ȷ�Ӧת�Ƶ�������Ϊ0.2NA |
| B��0.1mol�İ���(P4)������������Ĺ��ۼ�����Ϊ0.4NA |
| C����״���£�2.24LCl2ͨ������H2O��NaOH��Һ��ת�Ƶĵ�������Ϊ0.1NA |
| D���ھ���ͭ����ͭ�Ĺ����У�����������ͭ32gת�Ƶ�������ΪNA |
ij�ᾧˮ����Ļ�ѧʽΪR?nH2O������Է�������ΪM��25��ʱ����a g�þ�������b g H2O��ǡ���γ�V mL������Һ�����б���ʽ��ȷ����
A��������Һ���ʵ���Ũ��Ϊ�� 1000 a(M -18n) mol/L 1000 a(M -18n) mol/L |
B��������Һ���ʵ���������Ϊ�� |
C��25��ʱR���ܽ��Ϊ�� g/100 g H2O g/100 g H2O |
D��������Һ���ܶ�Ϊ�� g/mL g/mL |