��Ŀ����
����Ŀ���������ڱ���Ԫ��ԭ�ӽṹ���ʻش��������⣺
(1)Cԭ�Ӽ۲���ӵĹ������ʽΪ___________����̬Asԭ���У��������ռ�ݵ�����ܼ��ĵ���������ͼΪ___________�Ρ�
(2)��֪�ȵ�����������ƵĽṹ�ͻ�ѧ��������O22+��Ԫ��N�ĵ��ʻ�Ϊ�ȵ����壬��O22+�ĵ���ʽΪ___________��
(3)NH3�����ڶ����Ԫ�������γ�������CuSO4��Һ�м��������ˮ���õ�����ɫ��Һ�������м����Ҵ���������ɫ���壬�����Ҵ�������___________���þ���Ļ�ѧʽΪ___________��
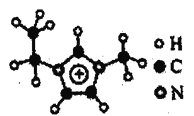
(4)��ͼEMIM+�����У�̼ԭ�ӵ��ӻ��������Ϊ___________�������еĴ�м����÷��Ŧ�nm��ʾ������n���������γɴ�м���ԭ������m���������γɴ�м��ĵ�����(�籽�����еĴ�м��ɱ�ʾΪ��66)����EMM+�����еĴ�м�Ӧ��ʾΪ___________��
(5)NiO����ṹ��NaCl���ƣ�������Ni2+λ���ڶ�������ģ�����O2��λ����___________����֪�����ܶ�Ϊdg/cm3��N2+ԭ�Ӱ뾶Ϊxpm��O2��ԭ�Ӱ뾶Ϊypm�����ӵ�������ֵΪNA����þ�����ԭ�ӵĿռ�������Ϊ___________(�г������ļ���ʽ)��
���𰸡�![]() ����
���� ![]() ��С�ܼ����ԣ����;����ܽ�� [Cu��NH3��4SO4]H2O sp2��sp3
��С�ܼ����ԣ����;����ܽ�� [Cu��NH3��4SO4]H2O sp2��sp3 ![]() ���ĺ�������
��������� ![]() ��100%
��100%
��������
(1)���ݻ�̬̼ԭ�ӵĺ�������Ų�ʽ��д���۲���ӵĹ������ʽ����̬Asԭ�Ӻ�������Ų�ʽΪ1s22s22p63s23p63d104s24p3������ܼ�Ϊ4p��
(2)���ݵȵ�����Ľṹ���ƣ�O22+�ĵ���ʽ��N2���� �����1��O22+�����������õ��Ӷԣ�
(3)��ˮ������ͭ��Ӧ����������ͭ��ɫ����������ˮ����ʱ����ˮ��������ͭ��Ӧ���ɿ����Ե�ͭ�����������������ܽ�õ�����ɫ������Һ���漰�����ӷ���ʽΪ��Cu2++2NH3H2O�TCu��OH��2��+2NH4+��Cu��OH��2+4NH3�T[Cu��NH3��4]2++2OH���ܷ�ӦΪ��Cu2++4NH3H2O�T[Cu��NH3��4]2++4H2O��������������ԭ�������Է��������ڼ����ܼ����Ҵ����Ӽ��Ա�ˮ���Ӽ������������Ҵ������ܼ��ļ��ԣ��Ӷ���С���ʵ��ܽ�ȣ������������Һ�м���һ�����Ҵ���������[Cu��NH3��4]SO4H2O���壻
(4)���ݷ��ӽṹ��֪�����������̼ԭ�Ӿ��γ�4���Ҽ����µ��Ӷԣ�����Ϊsp3�ӻ������ڵ�����̼ԭ�Ӿ��γ�3���Ҽ��������γɴ�м����µ��Ӷԣ�����Ϊsp2�ӻ������ڵĴ�м�������Cԭ�Ӻ�����Nԭ���γɣ�����ÿ��̼ԭ�ӹ���1�����ӣ�ÿ����ԭ�ӹ���1�����Ӷԣ���һ����λ������ɣ�˵������ʧȥ��һ�����ӣ�����6�������γɴ�м���
(5)��ΪNaCl����ṹ����������������������У�����NiO����ṹ����������������Ҳ��������У�������Ni2+λ���ڶ�������ģ�����O2��λ�����ĺ������ģ����ݾ����ܶȼ��㾧�����������Ni2+��O2����ԭ�Ӱ뾶��������ռ�õ����������ռ�õ�����Ⱦ�������Ϳɼ����������ԭ�ӵĿռ������ʡ�
(1)���ݻ�̬̼ԭ�ӵĺ�������Ų�Ϊ1s22s22p2����۲���ӵĹ������ʽΪ![]() ����̬Asԭ�Ӻ�������Ų�ʽΪ1s22s22p63s23p63d104s24p3������ܼ�Ϊ4p��������ܼ��ĵ���������ͼΪ�����Σ�
����̬Asԭ�Ӻ�������Ų�ʽΪ1s22s22p63s23p63d104s24p3������ܼ�Ϊ4p��������ܼ��ĵ���������ͼΪ�����Σ�
�𰸣�![]() ����
����
(2)���ݵȵ�����Ľṹ���ƣ�O22+�ĵ���ʽ��N2���ƣ����1��O22+�����������õ��Ӷԣ�O22+�ĵ���ʽΪ![]() ��
��
�𰸣�![]()
(3)��ˮ������ͭ��Ӧ����������ͭ��ɫ����������ˮ����ʱ����ˮ��������ͭ��Ӧ���ɿ����Ե�ͭ�����������������ܽ�õ�����ɫ������Һ���漰�����ӷ���ʽΪ��Cu2++2NH3H2O�TCu(OH)2��+2NH4+��Cu(OH)2+4NH3�T[Cu(NH3)4]2++2OH-���ܷ�ӦΪ��Cu2++4NH3H2O�T[Cu(NH3)4]2++4H2O��������������ԭ�������Է��������ڼ����ܼ����Ҵ����Ӽ��Ա�ˮ���Ӽ������������Ҵ������ܼ��ļ��ԣ��Ӷ���С���ʵ��ܽ�ȣ������������Һ�м���һ�����Ҵ���������[Cu(NH3)4]SO4H2O���壻
�𰸣���С�ܼ����ԣ����;����ܽ�� [Cu(NH3)4SO4]H2O
(4)���ݷ��ӽṹ��֪�����������̼ԭ�Ӿ��γ�4���Ҽ����µ��Ӷԣ�����Ϊsp3�ӻ������ڵ�����̼ԭ�Ӿ��γ�3���Ҽ��������γɴ�м����µ��Ӷԣ�����Ϊsp2�ӻ������ڵĴ�м�������Cԭ�Ӻ�����Nԭ���γɣ�����ÿ��̼ԭ�ӹ���1�����ӣ�ÿ����ԭ�ӹ���1�����Ӷԣ����д�һ����λ������ɣ�����6�������γɴ�м�����˸÷����еĴ�м��ɱ�ʾΪ![]() ��
��
�𰸣�sp2��sp3 ![]()
(5)��ΪNaCl����ṹ����������������������У�����NiO����ṹ����������������Ҳ��������У�������Ni2+λ���ڶ�������ģ�����O2��λ�����ĺ������ģ�
ÿ�������к���Ni2+��8��1/8+6��1/2=4������O2����1+12��1/4=4��M��NiO��=75g/mol
���ݾ����ܶȼ��㾧�������V0=![]() =
=![]() cm3
cm3
����Ni2+��O2����ԭ�Ӱ뾶��������ռ�õ������V1=4��4/3��![]() ����x��10-10��3+4��4/3��
����x��10-10��3+4��4/3��![]() ����y��10-10��3=
����y��10-10��3=![]() ��
��![]() ��10-30����x3+y3��cm3��������ԭ�ӵĿռ������ʣ�
��10-30����x3+y3��cm3��������ԭ�ӵĿռ������ʣ�![]() ��100%=
��100%=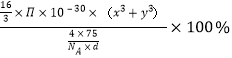 =
= ![]() ��100%��
��100%��
�𰸣����ĺ������� ![]() ��100%
��100%