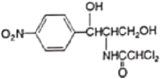
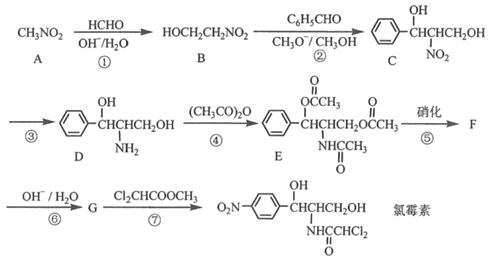
��Ŀ����
����Ŀ�����λ�����Ǧ(3PbO��PbSO4��H2O��������ˮ)��Ҫ�����ڲ����ľ�����ϩӲ�ʹܡ�ע�������Ʒ��Ҳ������������������Ʒ����Ǧ��(��Ҫ�ɷ�ΪPbO��Pb��PbSO4��)Ϊԭ���Ʊ����εĹ���������ͼ��ʾ��

��֪��Ksp(PbSO4)=1.82��10��8��Ksp (PbCO3)=1.46��10��13
��ش��������⣺
(1)����̼������Һ��PbSO4ת��ΪPbCO3��ת���ķ���ʽΪ��PbSO4(s)+CO32��(aq)=PbCO3(s)+SO42��(aq)��ͨ������(����2λ��Ч����)˵���÷�Ӧ�̶Ⱥܴ��ԭ��___________����һ�����У�Ҳ���Խ�̼������Һ��Ϊ̼��������Һ��д����̼��������Һʱ�����ӷ���ʽ��___________��
(2)����ͼ�ܽ������(g/100gˮ)������ҺI�õ�Na2SO4����IJ���Ϊ������Һ1��___________��___________�����Ҵ�ϴ�Ӻ����

(3)�����ۡ���������Ϊ����������ʣ��ɲ�ȡ�Ĵ�ʩ��___________(����д��һ��)������NO�����ӷ���ʽ��___________��
(4)����Һ2���п�ѭ�����õ�����Ϊ___________(�ѧʽ)��
�����ܡ���Ǧ��ʱ����Pb(NO3)2��Һ��0.02mol��L��1��H2SO4������������PbSO4����ʱ������Pb(NO3)2��Һ����С���ʵ���Ũ��Ϊ___________mol��L��1��
���𰸡��÷�Ӧƽ�ⳣ��K=1.2��105�������ܽ��� PbSO4+2HCO3-=PbCO3��s��+SO42-+H2O+CO2�� ���������£��ᾧ ���ȹ��� �ʵ����£��ʵ���������Ũ�ȣ���С����������С�Ⱥ����𰸾��ɣ� 3Pb+8H++2NO3-=3Pb2++2NO��+4H2O HNO3 3.64��10-6
��������
��Ǧ�ࣨ��Ҫ�ɷ�ΪPbO��Pb��PbSO4�ȣ�Ϊԭ���Ʊ����Σ���Ǧ���м�Na2CO3��Һ�ǽ�PbSO4ת����PbCO3��Na2CO3��aq��+PbSO4��s��=Na2SO4��aq��+PbCO3��s����������Һ���������Ҫ��Na2SO4������Na2CO3������Pb��PbO��PbCO3������������·�Ӧ��3Pb+8HNO3=3Pb��NO3��2+2NO��+4H2O��PbCO3+2HNO3=Pb��NO3��2+CO2��+H2O��PbO+2HNO3=Pb��NO3��2+2H2O����ת����Pb��NO3��2��Pb��NO3��2�м�ϡH2SO4ת����PbSO4�����ᣬ���˵���ҺΪHNO3����ѭ�����ã�������Ǧ�м����������ƺϳ����κ������ƣ���50�桫60�棬4PbSO4+6NaOH=3PbOPbSO4H2O+3Na2SO4+2H2O����Һ3��Ҫ�������ƣ�ϴ�ӳ�������õ����Σ��ݴ˷������
(1)��ӦPbSO4(s)+CO32��(aq)=PbCO3(s)+SO42��(aq)��ƽ�ⳣ��K=![]() =
=![]() =
=![]() =1.2��105�����ڷ�Ӧƽ�ⳣ������105�����Է�Ӧ���г̶Ⱥܴ����̼������Һ��Ϊ̼��������Һ��ע��д���ӷ���ʽʱ�������ʽ������Ӳ��ɲ����ӷ���ʽΪPbSO4+2HCO3-=PbCO3��s��+SO42-+H2O+CO2����
=1.2��105�����ڷ�Ӧƽ�ⳣ������105�����Է�Ӧ���г̶Ⱥܴ����̼������Һ��Ϊ̼��������Һ��ע��д���ӷ���ʽʱ�������ʽ������Ӳ��ɲ����ӷ���ʽΪPbSO4+2HCO3-=PbCO3��s��+SO42-+H2O+CO2����
�𰸣��÷�Ӧƽ�ⳣ��K=1.2��105�������ܽ��� PbSO4+2HCO3-=PbCO3��s��+SO42-+H2O+CO2��
(2)��ͼ�������֪���¶Ƚϸ�ʱ���������ƣ��¶Ƚϵ�ʱ���������ƽᾧˮ����������ҺI�õ�Na2SO4����IJ���Ϊ������Һ1�����������£��ᾧ�����ȹ��ˣ�ϴ�Ӿ���ʱ���Ҵ�ϴ�ӱ����γɽᾧˮ���
�𰸣����������£��ᾧ ���ȹ���
(3)��߷�Ӧ���ʿ��Դ����º��ʵ�����Ӧ��Ũ�ȣ�������巴Ӧ��Ӵ�����Ƕȿ��ǣ���Ϊ3Pb+8HNO3=3Pb��NO3��2+2NO��+4H2O����������NO�����ӷ���ʽ��3Pb+8H++2NO3-=3Pb2++2NO��+4H2O��
�𰸣��ʵ����£��ʵ���������Ũ�ȣ���С����������С�Ⱥ����𰸾��ɣ� 3Pb+8H++2NO3-=3Pb2++2NO��+4H2O
(4)Pb��NO3��2�м�ϡH2SO4ת����PbSO4�����ᣬ���˺���Һ�е�������ҪΪHNO3����ѭ�����������ܣ�
������Pb(NO3)2��Һ����С���ʵ���Ũ��Ϊx mol��L��1��Ksp(PbSO4)=1.82��10��8=C��Pb2+����C��SO42-��=![]() ��
��![]() �������x=3.64��10-6��
�������x=3.64��10-6��
�𰸣� HNO3 3.64��10-6

 ��������ܸ�ϰϵ�д�
��������ܸ�ϰϵ�д�����Ŀ����֪����Ӧ����I2(aq)+I-(aq)![]() I3-(aq) ��H1����Ӧ���� I3-(aq)
I3-(aq) ��H1����Ӧ���� I3-(aq) ![]() I2(aq)+I-(aq) ��H2����Ӧ�ٵĻ�ѧƽ�ⳣ��K1���¶ȵĹ�ϵ���±���
I2(aq)+I-(aq) ��H2����Ӧ�ٵĻ�ѧƽ�ⳣ��K1���¶ȵĹ�ϵ���±���
t/�� | 5 | 15 | 25 | 35 | 50 |
K1 | 1100 | 841 | 680 | 533 | 409 |
��ش�
(1)����Ӧ�ڵĻ�ѧƽ�ⳣ��ΪK2������ͬ�¶��£�K1��K2=____________��
(2)������Ӧ�ٵ���H1_______0(����>������=������<��)���������¶ȣ���I2���ܽ����ʻ�______(�����ӿ�����������������������)��
(3)���жϷ�Ӧ���Ѵﵽƽ���������_______________
A�������е�ѹǿ���ٸı� B����Һ����ɫ���ٸı�
C��I-Ũ�Ȳ��ٱ仯 D�����淴Ӧ���ʾ�Ϊ0
(4)ij�¶��£���Ӧ�ٵĻ�ѧƽ�ⳣ��Ϊ800���ڸ��¶��£���ס��ҡ������������зֱ����I2��I-�����������ʵ���ʼŨ�����£�
��ʼŨ��(mol/L) | �� | �� | �� |
c(I2) | 0.1 | 0.2 | 0.2 |
c(I-) | 0.2 | 0.1 | 0.2 |
��Ӧ����������_____________(������������������������)��ƽ��ʱI2��ת����������__________(������������������������)��